Introduction
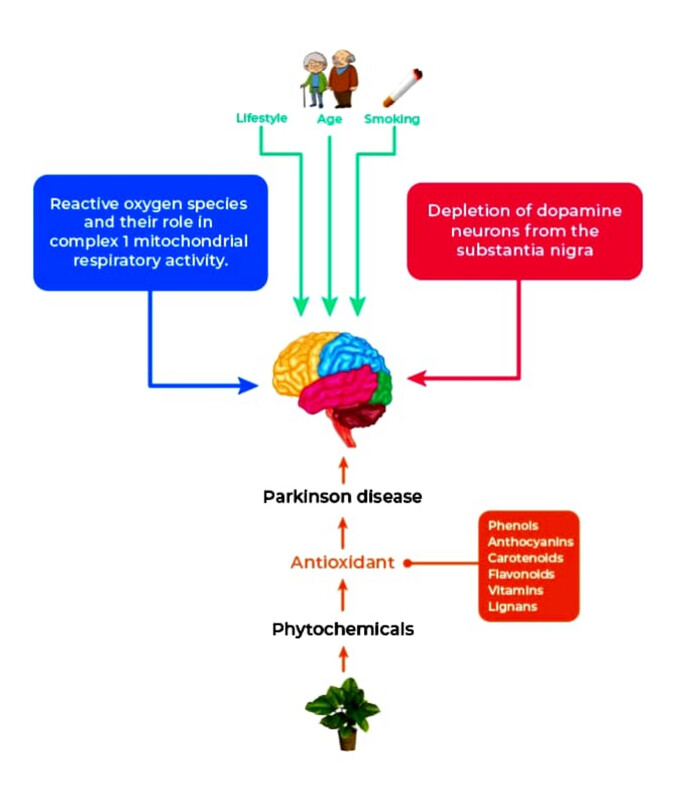
Parkinson’s disease (PD) is a frequently occurring neurological disorder that involves motor (tremor, postural instability, slow movement, and muscle rigidity) and non-motor (depression, cognitive dysfunction, hallucination, disorders of mood, apathy, and deep disruption) features. And also has illusions, hallucinations, and delusions (Beitz, 2014; Jankovic, 2008; Schneider, Iourinets, & Richard, 2017). The prevalence of PD is approximately around 7 to 10 million worldwide. However, when compared to other countries, PD prevalence in India is less. Though PD prevalence is less, the total burden of the disease is much higher as a result of the large population (Baiano, Barone, Trojano, & Santangelo, 2020).
Several biochemical aspects are found to be taking part in phenomena leading to mitochondrial dysfunction, excitotoxicity, gene mutations and neuroinflammation. Reactive oxygen species (ROS) overexpression, alterations to catecholamine metabolism, altered mitochondrial electron transporter chain functionality, and significant iron accumulation in the SN pars compacta (SNpc) are all factors that contribute to PD at the cellular level. The administration of levodopa is considered one of the cornerstones of the treatment of PD. PD and other age-related neurological illnesses like Alzheimer's disease are also being prevented or controlled by a number of nutritional supplements and natural antioxidants. Furthermore, plant-derived chemical compounds play an important role in preventing activities such as anti-inflammatory, antimicrobial, antioxidant, anti-aging, anti-diabetic, antidepressant, anticancer, and wound healing. The most abundant sources of these phytochemicals that play protective in many age-related diseases are fruits and vegetables (Slavin & Lloyd, 2012). Plants are full of compounds known as phytochemicals, which seem to have antioxidant properties.
The purpose of antioxidant supplements is to make up for nutritional deficiencies by consuming them in the right quantities, which consist of certain important compounds that support several biological processes. ROS and RNS (Reactive Nitrogen Species) caused damages can be prevented and repaired by free radical scavengers, which are the exogenous and endogenous antioxidants, and thereby, the immune defense will get enhanced and the risk of getting cancer and degenerative diseases gets lowered (Pham-Huy, He, & Pham-Huy, 2008). Additionally, chronic and degenerative disorders developments are influenced by oxidative stress. The antioxidants produced in our human body are either in situ or supplied externally via foods and nutrition supplements. α-lipoic acid and glutathione (GSH) are some of the powerful antioxidants that are naturally produced by our body’s cells. Vitamin C and E are those which are supplied through the food we consume. Additionally, antioxidants include flavonoids and carotenoids like flavonols, lycopene, quercetin, lutein, and anthocyanins present in cocoa, tomatoes, onion, apples, kale, and berries. A high dose of supplements may be harmful to a few individuals. Researchers have also studied antioxidants in their experiments, which showed that the antioxidants interacted with free radicals and stabilized them. Thus, this interaction prevents the free radicals from causing cell damage.
Antioxidant supplements
The PD progression was slowed down first by using antioxidants. Studies have been conducted to reveal the association of supplements and their antioxidant properties with the risk of PD. A balance between the creation of ROS and the body's antioxidant defense system is maintained by the human body's own internal oxidant system. Intake of a validated quantity of supplements is found to show a promising result for PD.
Polyphenols
Bioactive polyphenols are natural compounds whose structures consist of multiple phenolic groups, mostly found in different plant parts and plant products. Morbidity reduction and the development of cardiovascular and neurodegenerative diseases are slowed down with the help of polyphenols. The polyphenol’s biological properties show a strong relation to antioxidant activities. Inflammation, allergies, atherogenesis, thrombogenesis, and mutagenic effects can be prevented or reduced by them (Figure 1). Polyphenols daily intake ranging from 0.1 to 1.0g per day with the principal dietary may do benefit. The polyphenols of olive oil show a major hallmark by involving human health and metabolism (Rio et al., 2013; Ross & Kasum, 2002).
Effects of Olive oil and olive leaf extract
The olive oil extraction is done by separating oil from fruit pulp by crushing olives. Polyphenols, vitamins, and polysterols are found in olive oil even after the refining process (Gorzynik-Debicka et al., 2018). Triacylglycerols (TGA) contribute to the major group in olive oil. A variety of TGAs include monosaturated oleic acid as well as linoleic acid, palmitoleic acid, palmitic acid, and stearic acid. They are made up of glycerol esters with various fatty acids. Other than TGA, phenolic compounds, phytosterols, squalene, tocopherols, and derivatives of terpenic acids are also present in olive oil. Phenolic acids or alcohols, flavonoids, oleuropein derivatives, and lignans are the forms that can be exhibited by the phenolic compounds (Ramirez-Tortosa, Granados, & Quiles, 2006).
According to several types of research, olives are enriched with natural antioxidants that guard against conditions like cancer, neurological disorders, and coronary heart diseases. When compared to the other olive phenols, hydroxytyrosol (HT), which is prevalent in olives and virgin olive oil, has the strongest antioxidant activity because of its ability to donate electrons from its hydroxyl groups (Figure 2) (Fitó et al., 2005). The efficiency of HT’s radical scavenging activity was proved using research on rats-induced diabetes mellitus via alloxan. The neurochemical characteristics of HT point to the possibility that it may improve the neuroprotective effectiveness of monoamine oxidase (MAO) inhibitor therapy in PD patients. This natural phenol can readily permeate into the body's total amount of water and central neurons after being ingested. When administered orally, an increase in dosage linked with HT and its metabolites in brain tissues is found in experimental rats (Nicolaïew et al., 1998). Finally, HT is present in striatal microdialysate following systemic administration. (Goldstein et al., 2016) stated that autotoxic DOPAL production is effectively decreased by MAO inhibition. Treatment with MAO inhibitors and HT could mitigate the elevation in cerebrospinal fluid Cys-DA thus slowing the neural degeneration. The different activities of the olive oil and leaf extracts of olives are represented in Figure 3.
Ferulic acid
One of the most significant hydroxycinnamates found in red wine is ferulic acid (FA), a widely used herbal remedy and a naturally occurring compound with strong neuroprotective properties. The yellowish powder is how FA appears in its purest form. Many plants, including brown rice and whole wheat, have large amounts of FA in their leaves and seeds (Ojha, Javed, Azimullah, Khair, & Haque, 2015). The FA has provided protection in rotenone-intoxicated PD model through its anti-inflammatory and antioxidant effects. FA (40 mg/kg) had anti-parkinsonian effects in C57BL/6J mice by reducing apoptosis induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTPT), changing the ratio of apoptosis and neuroinflammation markers, and suppressing the activation of the microglial cell (Nagarajan, Chellappan, Chinnaswamy, & Thulasingam, 2015). When administered for two weeks, the active FA component Lithospermum officinale improved behavioral impairments and decreased neuroinflammatory responses in rats with MPTP-induced PD. Additionally, it has been claimed that FA can stop the aggregation of α-synuclein in the substantia nigra (Takahashi et al., 2015).
Another work using a rotenone-intoxicated PD model in D. melanogaster demonstrated the anti-parkinsonian effects of γ-oryzanol (steryltriterpenyl esters of FA). Dopamine, acetylcholine (AChE), SOD, glutathione-S-transferase, and catalase levels were also decreased. In this study, flies of both genders and ages from 1 to 5 days were exposed to rotenone for one week. Impairment in locomotor activity and reduction in AChE activity, mitochondrial function, and crossing numbers were observed (Figure 4). γ-oryzanol therapy increases motor function and repairs dopamine and oxidative parameters because it contains antioxidant components like ferulic acid (Thapliyal et al., 2021). The neurotherapeutic potentials of ferulic acid for PD progression management are highly supported by all of this research.
Flavonoids
In plants, the most generous class of phenols are flavonoids, such as flavanols, flavones, isoflavones, and anthocyanidins. Quercetin, hesperidin, anthocyanin, kaempferol, rutin, sibilinin, and naringin are the most studied flavonoids in relation to neurodegenerative diseases. The protecting impacts of flavonoids against PD models were demonstrated in cases that were both cell-based and animal studies and the decrease in oxidative stress and neuroinflammation, and prevention of the α-synuclein formation were also shown by flavonoids (Baptista, Henriques, Silva, Wiltfang, & Silva, 2014).
Hesperidin
Citrus fruits like lemon (Citrus limon), orange (Citrus sinuses) grapefruits (Citrus paradisi), pomelos (Citrus maxima), and bergamots (Citrus bergamia) consist of a rich source of citrus flavonoids. They include naringin, naringenin, quercetin, rutin, diosmin and hesperidin. Anti-apoptotic, anti-inflammatory, and antioxidant characteristics are some of the pharmacological effects shown by these flavonoids. Among these citrus flavonoids, hesperidin a flavanone glycoside possesses various biological and pharmacological effects (Hajialyani et al., 2019).
The structure of hesperidin includes an aglycon called hesperitin that is disaccharide-bonded to rutinose (Figure 5). Therefore, it is also considered as β-7-rutinoside of hesperetin (Garg, Garg, Zaneveld, & Singla, 2001). Through conducting hesperitin investigations, it was shown that mitochondrial apoptosis, neuroinflammatory, and transcription factors, which are controlled to reduce oxidative stress, are some of the triggering factors to induce neurodegeneration. Additionally, hesperidin showed modulatory activity on serotonergic and kappa-opioid receptors, which led to a reduction in depressive symptoms. By modulating neurotransmitter systems, hesperidin minimizes depression and cognitive function in mice. Dopamine and its metabolites being depleted hesperidin will inhibit homovanillic acid and 3,4-dihydroxyphenylacetic acid, and antioxidant activity can be produced by adjusting catalase, GSH, and glutathione peroxidase levels, inhibiting the production of ROS, and reducing GR activity (Antunes, Goes, Boeira, Prigol, & Jesse, 2014). Further, hesperidin promotes the efficiency in locomotion, and lipid peroxidation (LPO) inhibition in PD models, attenuating hypercholesterolemia and ameliorating DNA damage in chlorpyrifos-induced models of PD. Hesperidin is also thought to protect neurons by controlling other neurotransmitters like norepinephrine, epinephrine, and serotonin. Pro-inflammatory cytokines, COX-2, and inducible nitric oxide synthase (iNOS) levels as well as glial fibrillary acidic protein are reduced on consumption of hesperidin (Sakata, Hirose, Qiao, Tanaka, & Mori, 2003).
The current drug, L-Dopa is found to be less effective when compared to the activity of hesperidin. Hesperidin and L-Dopa when co-administrated enhanced the 6-hydroxydopamine (6-OHDA) bioavailability in PD animal model and also the inhibition of cytoplasmic vacuolation degeneration in the striatal and midbrain regions (Nagappan & Krishnamurthy, 2016). The gene expression of SNCA and LRRK2 which can cause PD was found to be reduced by the cooperative interactions of these two drugs. The same combination also potentiated the expressions of Parkin and PINK1 genes (Figure 6). Another combination, including hesperidin and Sinemet, one of the standard treatments for PD lessened its adverse effects on models of the disease caused by chlorpyrifos (Salem, El-Raouf, Saleh, & Shalaby, 2012).
Kaempferol
Kaempferol, a yellow-coloured compound found in plants is a tetrahydroxyflavone. Its 3,5,7 and 4’ positions consist of four hydroxy groups (Figure 7). Along with the ability to reduce neuronal damage and act as a defense mechanism against oxidative damage to the brain, antioxidant potential, and the capacity to pass the blood-brain barrier are all demonstrated. Recently studies have been carried out to test the protective potential of kaempferol in PD models like fruit flies (Drosophila melanogaster) and mice (Mus musculus). Those studies reveal that kaempferol has the capacity to lessen oxidative stress, suppress dopamine neuron damage, and improve cognitive performance (Jin, Zhang, & Wang, 2023).
The hallmarks of age-related neurodegenerative disorders include cell death and neuronal dysfunction. Exposure to kaempferol displayed its anti-apoptotic role like dose-dependent reduction in the caspase-9,3 activity and greyscale values. Kaempferol had the ability to decrease the influence of rotenone by reducing the cell death to half the number, resulting in the inhibition of caspase-9/3. Also, an increase of TH immune-positive cells was shown in PD flies. Kaempferol reduces oxidative stress and is involved in neuron protection by combining free radicals (Filomeni et al., 2012).
In many studies, a dose-dependent rise in the odor choice index (OCI) when exposed to kaempferol at various doses was displayed in PD flies which had a reduced odor choice index. Their findings suggest that the mushroom body, which is the region of the D. melanogaster brain that controls the olfaction is affected through the gene expression and the Lewy body formation, due to the expression of SNCA panneurally (Rahul & H, 2021). D. melanogaster olfaction is lost as a result of the development of these Lewy bodies, which harm the neurons in the mushroom body. The Lewy body formation and neuronal damage altogether enhance neurodegeneration. By minimizing the neuronal oxidative stress caused by exposing flies to kaempferol, this damage to the neurons was minimized, and as a result, the OCI in the flies increased.
The courtship behavior of D. melanogaster involves autonomic, sensory, and motor systems and can be affected by the SNCA gene expression. A significant increase in the courtship index (CI) was displayed by flies with PD when exposed to different kaempferol doses earlier showed a significant decrease in the CI. Additionally, the genetically modified flies which expressed A30P α-synuclein in their brain also showed a decline in the behavioural response (Figure 8) (Feany, 2000).
Anthocyanin
Anthocyanins, a natural compound in plants, belong to one of the subgroups of flavonoids. Numerous berry varietals and the red wine made from them contain larger concentrations of these cationic compounds (Mazza, 2007). The presence of color pigmentations in fruits is due to the presence of this compound. There are currently roughly 27 anthocyanin compounds known to exist in nature, with cyanidins, pelargonidins, delphinidins, petunidins, peonidins, and malvidins being the most prevalent. Studies have discovered that anthocyanin has a major role in some pathology such as anti-diabetic, and anti-inflammatory, and also has shown protection against neuro and cardiovascular diseases (Ali et al., 2018; Wallace & Giusti, 2014).
Anthocyanins have both intrinsic and extrinsic antioxidant properties. Because of their structure's capacity to transfer electrons or hydrogen, they can scavenge free radicals. One of the reasons for their neuroprotective capability is that they show great oxygen radical absorption capacity (Zhu, Cai, Yang, Ke, & Corke, 2010). Anthocyanins' inherent antioxidant action may be indirect and related to, (i) the rise in endogenous antioxidant enzyme activity, which can raise the antioxidant levels (Fig. 4): (ii) also by mediating activation of antioxidant response elements, which in turn activates the endogenous antioxidants (Nrf2): or (iii) inhibition of xanthine oxidase and NADOH oxidase for the reduction of endogenous ROS formation or by modification of mitochondrial respiration (Figure 9) (Ali, Rehman, Shah, & Kim, 2018; Steffen, Gruber, Schewe, & Sies, 2008).
GSH and coenzyme Q10 (CoQ10) like endogenous antioxidants, and superoxide dismutase (SOD) and catalase-like antioxidant enzymes, under normal conditions, are involved in mitochondrial respiration and can lead to detoxifying ROS and RNS. The decreased antioxidant enzyme performance and diminished levels of GSH and CoQ10 are some of the cases of neurodegeneration (Johnson, Wilson-Delfosse, & Mieyal, 2012; Niedzielska et al., 2016). ROS and RNS are accumulated and oxidative damages are caused when there is a loss of intrinsic antioxidant defense. The aforementioned pathways result in the production of free radicals, and changes in the maintenance of oxidative stress and cellular energy, all of which collectively cause cell death. Anthocyanins directly scavenge the damaging ROS and RNS due to the high ORAC value. Other than the flavonoid-quercetin, plum extract is the major constituent of anthocyanin with higher superoxide radicals scavenging capacity (Aimee, Ross, Khatter, Miller, & Linseman, 2017).
Many studies conducted using fruit extracts that are rich in anthocyanin and in vitro pure anthocyanins to demonstrate their potency to protect various cell lines of neurons from the toxicity of hydrogen peroxide. A study conducted with hydrogen peroxide-induced glial cells showed that the treatment with anthocyanin showed significant improvement in the cell's viability and oxidative stress index was induced along with apoptosis within the treated cells. Anthocyanin is believed to have the ability to scavenge free radical species like hydrogen peroxide, which can get elevated when mitochondrial dysfunction and glial inflammation occur, which here may be useful to reduce oxidative damage in neurodegenerative diseases. An in vivo investigation revealed that carbon tetrachloride can increase the generation of reactive species and oxidative damage in the organs of rats. After consuming anthocyanin-rich grape juice, rats' levels of oxidative damage markers like LPO and protein carbonylation significantly decreased (Dani et al., 2008).
Quercetin
Quercetin bioactive flavonoid compound with potent antioxidant properties and can be used as medicine. It is abundant in berries, apples, kale, onions, red grapes, broccoli, tea and also in red wine. Quercetin’s antioxidant activities affect GSH, ROS caused by the environment and toxicological factors, enzymatic activity, and signal transduction pathways (Xu, Hu, Wang, & Cui, 2019). Quercetin, a chemo-liable and thermo-liable compound can lower bioavailability at the target site (Cai, Fang, Dou, Yu, & Zhai, 2013). Therapeutically, quercetin has anti-diabetic, anti-oxidant, anticancer, and anti-inflammatory properties (David, Arulmoli, Parasuraman, & S, 2016). Studies using quercetin-treated animal models of neurodegenerative illnesses showed advancement in behavior as well as a decrease in apoptotic proteins and antioxidant mechanisms and elevated anti-apoptotic processes. Quercetin can act as a neuroprotective agent because of it is capacity to permeate the blood-brain barrier and exert antioxidant and anti-inflammatory effects. Through a covalent bond, quercetin binds to α-synuclein to produce adducts. After being oxidized by the dissolved oxygen, quercetin can produce a variety of compounds, including chalcanthite, benzyfuranone, quercetin chinone, and others (Costa, Garrick, Roque, & Pellacani, 2016).
Quercetin increases the GSH content reduces the free radical level and can prevent the oxidative damage induced by paraquat. When rat cortical cells were primarily cultured against oxidative neuronal injury, the neuropotential role of quercetin was observed. In research using a 6-OHDA-induced rat model, quercetin's anti-PD actions have been demonstrated through elevated levels of antioxidant enzymes in rats, such as GSH. In previous studies conducted on PD models, quercetin was demonstrated to have an inhibitory effect on the iNOS/NO system and pro-inflammatory markers (Haleagrahara, Siew, & Ponnusamy, 2013; Sriraksa et al., 2012) (Haleagrahara et al., 2013). (Karuppagounder et al., 2013) demonstrated quercetin's ability to prevent the loss of complex I activity in a PD model intoxicated with rotenone. Oral administration of nano-coated quercetin was able to protect the brain of rats and cells of the liver against arsenic-induced damage. These tests validated quercetin's capacity to protect the gastrointestinal tract and ensure safe transport to the intended brain region (Ghosh, Mandal, Sarkar, Panda, & Das, 2009). Using an emulsion-diffusion-evaporation technique, the same research team produced nano quercetin, which demonstrated greater availability in different regions of the brain, both in young and aged rats (Ghosh, Sarkar, Mandal, & Das, 2013).
Alkaloid
Trimethylxanthine (Caffeine)Alkaloids are the naturally occurring compounds. The main purine alkaloid, trimethylxanthine, source for many users is coffee (1,3,7-trimethylxanthine). It is the substance that is legally consumed the most and is also utilised as an ergogenic aid. It is discovered to be a potential contribution to easing PD symptoms. Further, it also has the property to reduce the risk factors involved in metabolic syndrome, like obesity (Sökmen et al., 2008). In addition to approved medication and therapies given to PD patients like dopamine replacement therapy, flavonoids and other substances derived from numerous plants have demonstrated their positive effects, mostly by controlling the increased oxidative stress and neuroinflammation. Trimethylxanthine showed a significant role in regulating the role of dopamine when compared with the different compounds used for the treatment of PD models. By elevating the dopamine level and suppressing the A2 adenosine receptors, trimethylxanthine has been proven to enhance the function of the dopaminergic system. Adenosine is found to suppress physiological arousal and neuronal excitability by inhibiting the neurotransmitters released from the brain. Further, adenosine receptor antagonism is considered to be primarily by the trimethylxanthine’s ergogenic mechanism (Trevitt, Kawa, Jalali, & Larsen, 2009).
(Ikram, Park, Ali, & Kim, 2020) studied the activity of trimethylxanthine in a PD model administrated with 6-OHDA. The outcome of the study revealed trimethylxanthine has the ability to decrease the histone deacetylase, tyrosine hydroxylase, TNF-α, and increase motor activity. The trimethylxanthine lowered the gamma-aminobutyric acid expression and boosted the paw strength of the mice induced with MPTP. In SH-SY5Y cells induced with MPP+, trimethylxanthine activated the P13K/Akt signaling and also regulated apoptotic cell death. Additionally, in α-synuclein-induced animals, chaperone-directed autophagy, expression of LAMP2A, and sequestosome 1 protein were reduced, and macroautophagy was balanced by greater development of the microtubule-related protein LC-3 (Luan et al., 2018).
Antioxidant vitamins
Investigations are done to find out the relationship between vitamins (vitamins C, E, B6, and B12, folate, and carotenoids) and PD (Zhao et al., 2019). It was discovered that vitamin B12 and folate have no effect on PD risk reduction (Shen, 2015). But in PD patients levels of vitamin were found to be less. However, it is suggested that the risk of PD development is lowered by intake of vitamin B6 via diet. Homocysteine, an amino acid, has been proven to have numerous neurotoxic effects, the intake of vitamin B-rich food can lower the plasma homocysteine content. There was no correlation between the dietary consumption of vitamin B12 and folate and the risk of PD. However, a vitamin B6 diet decreases the risk of PD. According to reports, vitamin B6 has antioxidant properties. Pyridoxine has been reported to have a singlet oxygen-quenching activity that is comparable to the highly strong antioxidant vitamins C and E. Additionally, dietary vitamin E had no impact on the likelihood of PD, but dietary vitamin C had the reverse effect (Miyake et al., 2011). Vitamin E was found to give a long-term treatment and use of levodopa could be delayed.
Vitamin B6 deficiency leads to high LPO in the liver and plasma, which will lead to some consequences such as a decrease in some antioxidant factors such as vitamin E, reduced and GSH levels. Pyridoxal phosphate preincubation of the endothelial cells for a half-hour revealed a considerable decrease in the superoxide and lipid peroxide levels (Mahfouz, Zhou, & Kummerow, 2009). Rats' livers and hearts will experience oxidative stress due to a vitamin B6 shortage; supplementing with vitamin B6 can help to lessen the severity of oxidative stress (Shen, 2015). In rats, homocysteine-induced atherosclerosis can be delayed by the antioxidant activity of vitamin B6. Furthermore, vitamin B6 supplementation was found to have anti-inflammatory and antioxidant activities in addition to the expected homocysteine-lowering effect in stroke illness (Ullegaddi, Powers, & Gariballa, 2004). Anther investigation made on PD with vitamins is tabulated in glutathione.
A common thiol tripeptide known as GSH is a crucial endogenous antioxidant in the brain that protects against oxidative stress-related ROS damage. A lower concentration of GSH is observed during the early stages of PD; no detectable amount of GSH is seen in the substantia nigra during the progression of the disease (Weber & Ernst, 2006). LPO and gamma-glutamyl transpeptidase activity are increased as a result of the decreasing amounts of GSH in both whole cells and mitochondrial fracrions (Bjørklund, Peana, Maes, Dadar, & Severin, 2021). Neuron loss is also brought on by an increase in oxidative stress brought on by low GSH levels.
Between its heavy and light chain subunits, glutamyl cysteine ligase is considered as rate-limiting stage of GSH production and has both disulfide and noncovalent linkages. It is a dimeric protein made up of a modulatory (GCLc) and a catalytic (GCLc) subunit (GCLm). When GCLc/GLCm expression is silenced, it causes the progressive degeneration of nigral dopamine neurons, and when it is overexpressed, it causes abnormal glutathiolation of neuronal proteins and loss of neurons. According to research, PD patients have very low levels of GCL activity, which is necessary for detoxification, GSH production, and GSH regeneration. Neuronal death (tyrosine hydroxylase) induced by MPTP in the SNcp is amplified by 1-buthionine sulfoximine, an irreversible inhibitor of GCL, depleting GSH (Bharath, Hsu, Kaur, Rajagopalan, & Andersen, 2002). A subcutaneous injection of BSO reduces the effects of GSH depletion-induced mitochondrial brain injury by promoting the overproduction of transgenic copper, zinc-SOD. Long-term dietary folate/methyl deficiency may also result in a decreased GSH/GSSG ratio in rat brain tissue, which is followed by irreversible oxidative DNA damage. The glutamate neurotoxicity can also be related to reduced uptake of cysteine (Bagnyukova, Powell, Pavliv, Tryndyak, & Pogribny, 2008).
Cellular susceptibility to free radicals has been demonstrated by the lowering of GSH activity by ethacrynic acid (Somberg & Molnar, 2009). In astrocyte culture, GSH deficiency may cause extracellular glutamate to accumulate to a large degree (Mcnaught & Jenner, 2000) and astrocyte GSH levels decreased dose-dependently is caused due to glutamate exposure. Furthermore, astrocytes release GSH into extracellular space following glutamate stimulation and this provides a protective and adaptive response. According to a recent discovery, selenium-related reduction in glutamate excitotoxicity is independent of GSH content modification and may control the relationship between neurodegeneration and neuroinflammation (Savaskan et al., 2003). Vitamins associated with PD are mentioned in Table 1.
Table 1
Investigation of Parkinson's by using vitamins.
Vitamin | Study type | Number of controls/ patients used | Conclusion |
|---|---|---|---|
Vitamin B3 | Swedish case-control research | 263/113 | A diet high in niacin can lower the risk of PD (Fall, Fredrikson, Axelson, & Granérus, 1999) |
German case-control study | 342/342 | PD patients consume less niacin than healthy individuals (Hellenbrand et al., 1996) | |
Japan case-by-case analysis | 62 PD | Patients with severe PD have significantly reduced amounts of vitamin C in their lymphocytes (Ide et al., 2015) | |
An American case-control study | 16/27 | PD groups had higher levels of vitamin C (King, Playfer, & Roberts, 1992) | |
Vitamin E | Sweden's prospective study | 1329 cases were PD in total 84,774 subjects | Dietary consumption of vitamin E and the incidence of PD in women were negatively correlated (Yang, Wolk, Håkansson, Pedersen, & Wirdefeldt, 2017) |
Japan's case-control study | 368/249 | Significantly lowering the risk of PD was vitamin E (Miyake et al., 2011) | |
A prospective US study | 371 cases were PD in 1,24,221 total subjects | Increasing your vitamin E intake can reduce the chances of developing PD (Zhang et al., 2002) | |
Vitamin D | An observational, prospective study in Korea | 39 PD cases | Vitamin D may have an effect on PD patients' olfactory impairment (Kim et al., 2018) |
An England Prospective Observational Study | 94/145 | Comparing the controls and PD patients had lower concentrations of serum 25 (OH) D concentrations which corresponds to the severity of motor symptoms (Sleeman et al., 2017) | |
Chinese case-control research | 199/201 | Sunlight exposure and serum 25(OH)D had an antagonistic relationship with the development of PD (Wang, Yang, Yu, Shao, & Wang, 2016) | |
Chinese case-control research | 120/229 | The severity of PD may be inversely related to the 25(OH) D levels (Liu & Zhang, 2014) | |
Hungary's case-control study | 109/100 | The connection between the FokI C allele and PD is shown in this study (Török et al., 2013) |
Coenzyme Q10
Coenzyme Q10 is a cofactor for the electron transport chain for mitochondrial complex I and II and antioxidant (Mantle, Heaton, & Hargreaves, 2021). It is possible to mimic pathological and clinical characteristics of PD in animal models by inhibiting the complex I activity in the electron transport chain using MPTP. Synthetic heroin is yet another name for MPTP. The enzyme MPP+ converts this MPTP into the neurotoxin MAO B. By inhibiting mitochondrial respiratory chain complex I activity, this causes rats and primates to experience MPP+, a condition resembling PD (Schapira, 2011). Although oxidative stress is regarded as a major contributing element, the exact cause of cerebral MRC complex I activity is unknown. In PD patients, there was a lack of cerebral CoQ10 status as well as signs of decreased MRC complex I activity. In postmortem examinations of the substantia nigra and platelets from PD patients, there is decreased complex I activity (Benecke, Strümper, & Weiss, 1993).
In its reduced form CoQ10 serves as a powerful antioxidant and provides protection to the cells from oxidative stress. All the above findings state that the supplementation of CoQ10 can be a promising benefit for PD patients. It was also found that oral CoQ10 supplementation in MPTP-treated one-year-old mice reduced dopaminergic axon loss when compared with a standard diet. CoQ10 in vitro models showed protection against rotenone that also inhibits complex I activity. Patients with PD didn't experience many benefits from the anti-parkinsonian drug with CoQ10 (Negida et al., 2016). However, high doses may be beneficial for people with PD (Manzar, Abdulhussein, Yap, & Cordeiro, 2020).
Conclusion
Although the root cause of PD is yet unknown, several evidences have shown that oxidative stress plays a pivotal part in the disease. Parkinson’s is associated with mitochondrial dysfunction, ROS production, optical density, protein aggregation, and cell death. Oxidative stress and mitochondrial dysfunction are significant events in the neurodegeneration associated with PD by interpreting the functional characteristics of alterations in LRRK2, PINK1, DJ-1, PARKIN, and SNCA. Different oxidative stress biomarkers, including the protein DJ-1, homocysteine, retinoic acid, uric acid, 8-hydroxydeoxyguanosine, CoQ10, and LPO by-products, are intended for improvising diagnosis of PD at early stages, forecast its progression, and track the effectiveness of treatment. Treatments for this disease with natural antioxidants mainly polyphenols, alkaloid trimethylxanthine, antioxidant vitamins, GSH, and coenzyme Q10 through dietary supplements is an alternative option to synthetic compounds that have clinically proven toxicity. Antioxidants and supplements have a potentially limited role, notwithstanding this. Dietary consumption of flavonoids such as hesperidin, kaempferol, anthocyanin, quercetin, and ferulic acid may be useful for slowing down the onset or progression of PD. Among the various compounds present in coffee, trimethylxanthine has displayed protective activity against dopaminergic neurotoxicity in animal models. When present in high concentrations in serum, a fat-soluble antioxidant vitamin like vitamin E can lower the risk of PD. In senior PD patients, vitamin C has been proven to enhance levodopa absorption. Furthermore, cellular GSH depletion within SN dopaminergic neurons affects various cellular processes including mitochondrial function contributing to neurodegeneration in the disease. In the case of CoQ10, functional decline slowing and providing of symptomatic benefit for PD patients will not be shown.
Author contributions
This research article is done by collaborating with the authors. Conceptualization, A.V.A and B.B.; Writing original manuscript, D.A, B.B.; Selected bibliographic sources and content: D.A., S.P., K.B., K.P., A.M., T.S., M.K.; Figures, K.P., A.M. D.A.; Writing-review and editing, B.B., A.M., S.P., A.V.A.,; All the authors revised and approved the final article.
Conflicts of interest
Given his role as Associate Editor, Balamuralikrishnan Balasubramanian has not been involved and has no access to information regarding the peer review of this article. Full responsibility for the editorial process for this article was delegated to Co-Editor Carlos L. Cespedes Acuña. The authors hereby declare that they have no conflict of interest and have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
6-OHDA - 6-Hydrodopamine
AChE - Acetylcholine
Akt - Akinase (PRKA) C-Terminal Domain
BSO - Buthionine Sulpoximoine
CI - Courtship Index
CoQ10 - Coenzyme Q10
COX-2 - Cyclooxygenase 2
DJ-1- Protein Deglycase/Parkinson Disease Protein 7
DNA - Deoxyribonucleic Acid
FA- Ferulic Acid
GCLc - Glutamate-Cysteine Ligasse Catalytic Subunit
GCLm- Glutamate-Cysteine Ligasse Catalytic Subunit
GSH - Glutathione
GSSG -Glutathione Disulfide
HT- Hydrotyrosol
iNOS - Inducible Nitric Oxide Synthase
LAMP2A - Lysosome-Associated Membrane Protein
LPO - Lipid Peroxidation
LPO - Lipid Peroxidation
LRRK2 - Leucin-Rich Repeat Kinase 2
MAO - Monoamine oxidase
MPP+ - 1-Methyl-4-Phenylpyridium
MRC - Mitochondrial Respiratory Chain
MTPT - 1-methyl-4-Phenyl-1,2,3,6-Trahyropyridine
NO -Nitric Oxide
NrF2 - Nuclear Factor Erythroid 2-related Factor 2
OCI - Odour Concentration Intensity
ORAC - Oxygen Radical Absorbance Capacity
P13K - Phosphoinositide 3-kinase
PD - Parkinson’s disease
PINK1 - PTEN Induced kinase 1
RNS - Reactive nitrogen Species
ROS - Reactive Oxygen Species
SH - SY5Y- SK-N-SH Neuroblastoma Cell Line
SNCA -Synuclein Alpha
SNpc - Substantia Nigra Pars Compacta
SOD - Superoxide Dismutase
TGA - Triaglycerols
TH - Helper T cells
TNF α - Tumor Necrosis Factor Alpha