Introduction
In agriculture, pesticides are substances consisting of one or more chemical compounds that are typically sprayed on crops to control or eliminate weeds, fungi, rodents, and other pests (Zamani, 2013). The term "pesticide" broadly refers to various categories of substances, including insecticides, herbicides, fungicides, rodenticides, wood preservatives, garden chemicals, and household disinfectants, which are used to either kill or protect against pests (Eldridge, 2008). But the residue they leave may seriously endanger both the environment and human health (Kaur et al., 2024). Self-poisoning from pyrethroid and organophosphorus pesticides is a major clinical and public health concern in many rural Asian countries, such as India (Fernando, 1995; Jeyaratnam, Lun, & Phoon, 1987). Approximately 60% of the region's annual estimated 500,000 self-harm deaths are attributed to pesticide poisoning (Gunnell & Eddleston, 2003). Numerous studies have estimated that approximately 200,000 annual deaths attributed to pesticides account for around two-thirds of these fatalities (Mew et al., 2017). The pyrethroid pesticides are synthetic compounds produced to mimic the insecticidal effects of pyrethrins, which are naturally occurring compounds and are extracted from chrysanthemum flowers. The symptoms of pyrethroid exposure that are predominantly caused by neurotoxicity are tremors, convulsions, and respiratory distress (Shafer, Meyer, & Crofton, 2005). Tetramethrin (Figure 1a), a synthetic pyrethroid, is commonly employed due to its effectiveness against a broad spectrum of insects. It acts on the nervous system of insects, leading to paralysis and death. Despite its efficacy, concerns regarding its persistence in the environment and potential health impacts necessitate accurate detection methods of Zhang, Wang, Chi, Liu, and Hong (2008). Pesticides classified as organophosphorus (OPs) are known for their strong acetylcholinesterase (AChE) inhibitory properties. Acetylcholine accumulates in the body as a result of this inhibition, overstimulating the neurological system. Severe clinical indications of OP poisoning might include convulsions, respiratory failure, and twitching of the muscles (Brown & Brix, 1998; Figueiredo, Apland, Braga, & Marini, 2018). Dimethoate (Figure 1b), on the other hand, is an organophosphate pesticide that inhibits the enzyme acetylcholinesterase, leading to neurotoxic effects in both target pests and non-target organisms, including humans. The detection of these pesticides in food and biological samples is critical for ensuring food safety and compliance with regulatory standards (Dey & Saha, 2014; Li et al., 2020; Liu et al., 2013; Yan et al., 2021). Ultrasonic extraction has emerged as a transformative technique in analytical chemistry, particularly for the detection of pesticides in various matrices (Syahir, Sulaiman, Mel, Othman, & Sulaiman, 2020). This method leverages the principles of ultrasound to facilitate the extraction of target compounds, enhancing the efficiency and effectiveness of traditional extraction techniques (Carreira-Casais et al., 2021; Kumar, Srivastav, & Sharanagat, 2021). The focus of this study is on the development and optimization of an ultrasonicator-aided cartridge-based extraction method for the detection of two specific pesticides: the pyrethroid tetramethrin and the organophosphate dimethoate, in simulated biological matrices such as gastric fluid and urine via Ultra-Performance Liquid Chromatography tandem Mass Spectrometry (UPLC-ESI-MS/MS).
Materials and Methods
Chemicals and Materials
For this work, only analytical-grade chemicals and reagents were used. Pestanal standard samples of TMT and DMT, were purchased from Merck Life Sciences, chemicals used in preparation of simulated matrices were purchased from Sisco Research Laboratories Pvt. Ltd. (SRL), India, and solvents like methanol, acetonitrile and formic acids were purchased from Thomas Baker (Chemicals) Pvt. Ltd. India, milli-Q water used from Merk-millipore Bangalore India, HLB cartridges were purchased from Waters India Pvt Ltd.
Preparation of simulated gastric fluid and urine samples
Simulated gastric fluid was carefully prepared according to the United States Pharmacopeia (USP) guidelines (Test Solutions, USP 35, NF 30, 2012) and the method described by (Wang, Yadav, Smart, Tajiri, & Basit, 2015). The preparation involved dissolving 0.03 M sodium chloride, 0.084 M hydrochloric acid, and 0.32% (w/v) pepsin in milli-Q water, with the final volume adjusted to 100 mL. For simulated urine samples, the procedure outlined by (Stolarz et al., 2005) was followed. Organic components were prepared using 16 g/L urea, 9.60 g/L chlorine, 5.40 g/L sodium, 1.35 g/L sulfate, 0.65 g/L magnesium, 0.20 g/L calcium, and 0.20 g/L potassium, all dissolved in 1.0 L of milli-Q water and adjusted to a pH of 6, as specified by (Sarigul, Korkmaz, & Kurultak, 2019).
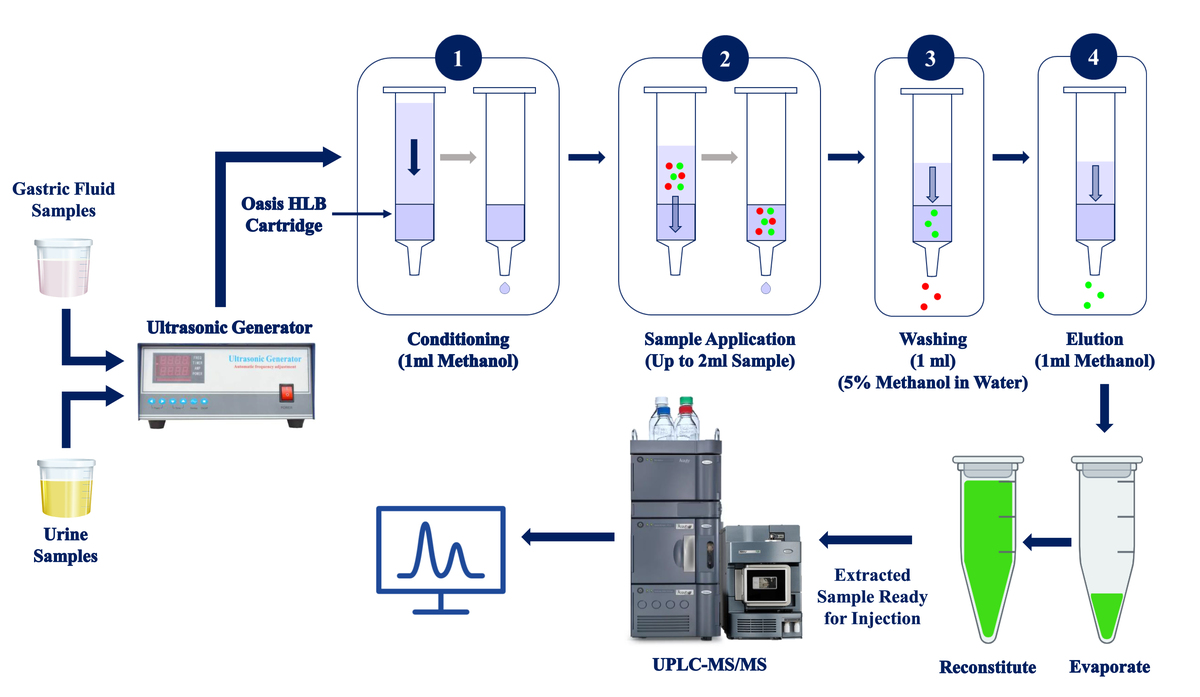
Solid Phase Extraction (SPE)
Column conditioning, sample loading, washing, and elution are the four main steps in the solid-phase extraction (SPE) process, which partitions key compounds between a solid phase (sorbent) and a liquid phase (sample) (Biziuk & Żwir-Ferenc, 2006). These steps are always applicable, regardless of the type of sorbent used, the format (such as cartridges, discs, 96-well plates, or pipette tips), or whether the process is automated (online SPE) or manual (offline SPE) (Badawy, El-Nouby, Kimani, Lim, & Rabea, 2022). Since SPE can isolate trace compounds simultaneously, reduce matrix interferences, work quickly and easily, require fewer organic solvents, and has a high preconcentration factor, it is preferred over alternative sample preparation methods.
Oasis HLB Cartridge-Based SPE
Oasis HLB is a sorbent known for its Hydrophilic-Lipophilic Balance (HLB), a feature that provides the required selectivity and sensitivity. This characteristic enables the rapid and reliable development of sample preparation techniques, resulting in high and consistent recoveries. Notably, these techniques are highly compatible with UPLC-MS/MS analysis.
Preparation of Calibration standards and quality control standards
Primary stock solution of TMT and DMT was prepared individually by dissolving 1000 µg in 1 mL of the solvent at a concentration of 1000 µg/ml. The primary stock solution of TMT and DMT was successively diluted in methanol to prepare the calibration standards at seven different concentrations (2.5, 5,10, 20, 40, 80, and 160 ng/mL) of TMT and DMT (Gualdesi, Esteve-Romero, Briñón, & Raviolo, 2013).
Sample Preparation
A total of 5 mL of liquid samples with 1% NaCl (w/v) were carefully transferred into a 10.0 mL volumetric flask. The sample solution was then quickly mixed with a blended mixture which contained 5 mL of the extraction solvent 1-dodecanol and 5 mL of methanol as the disperser solvent. The resulting mixture was slightly stirred for one minute, or until a cloudy solution formed inside the test tube with finely scattered droplets. Afterwards a three-minute ultrasonication, the resulting mixture was centrifuged at 4000 rpm for 5 minutes.
The test tube was refrigerated for 5 minutes to solidify the droplets. Once solid, the droplets were solidified. it was removed with nippers and transferred to a room-temperature conical flask, where it gets quickly melted. Afterwards, 1.0 mL of the melted extractant was added to prepare the Oasis HLB cartridge for extraction. The vacuum of the manifold was set to 5’ Hg, conditioned with Methanol, and equilibrated with water. After adding the solvent, the vacuum flow was stopped. The diluted sample was loaded, and then the vacuum flow was increased and washed with 5% methanol. The waste was discarded, and the analyte was eluted out with 100% methanol and collected. Finally, evaporated, reconstituted, and diluted the sample for injection into the UPLC-MS/MS instrument.
Data acquisition
The qualitative and quantitative analysis was performed on a hyphenated H-Class Acquity UPLC system coupled with a Waters Xevo Tandem Quadrupole detector (Waters Co., Milford, MA, USA) interface via electrospray ionization (ESI) source. The chromatographic separation was achieved on Waters BEH C18 (50 × 2.1 mm, 1.7 μm) reverse-phase column at the constant flow rate of 0.5 mL/min and 1µL of injection volume. The mobile phase consisted of acetonitrile (A) and 0.1% formic acid (prepared in 95:5 v/v water/acetonitrile) buffer (B) and delivered under an isocratic program (80:20 for A: B over 10 minutes). The applied column and the auto-sampler temperature were set at 35°C and 20°C respectively. MS/MS experiments were conducted in ESI positive ion mode to obtain multiple reaction monitoring (MRM) for TMT and DMT. The ESI parameters were set with a capillary voltage of 3.5 kV and a cone voltage of 30 V. Nitrogen was used as both the nebulizing and drying gas at flow rates of 50 L/h and 950 L/h, respectively. The source and desolvation temperatures were maintained at 120°C and 350°C. Argon gas was used for collision-induced dissociation (CID). Data acquisition and data reduction were carried out using MassLynx V4.2 software (Table 1).
Table 1
Data acquisition parameters for UPLC-MS/MS system.
Results and Discussion
Prerequisites for Implementing LC-MS/MS
Spectrometric and chromatographic parameters were optimized for the detection of TMT and DMT via directly injecting spiked standard solutions into the UPLC-MS/MS system. The two distinct product ions have been determined by applying various collision energy voltages after the initial detection of each precursor ion. The qualifier ion and quantifier ion were selected for the two transitions, respectively. Finally, dual timing and multiple reaction monitoring (MRM) transitions were used to adjust the parameters as shown in Figure 9; Figure 5 respectively. In addition to MS parameters, the mobile phase's composition which included the buffer solution's pH and concentration which had a significant impact on ionization efficiency in MS and peak shape in chromatography, both of which improved quantification specificity. The TMT and DMT retention times for gastric fluid and urine samples were determined under optimal UPLC conditions as shown in Figure 6; Figure 2 respectively. Using a tuning solution containing 100.0 ng/mL, the MS parameters were adjusted for both positive and negative ionization modes. The positive ionization mode exhibited significantly higher sensitivity for detecting TMT and DMT, with reduced background noise compared to the negative mode. The mass transitions of the precursor ions for TMT and DMT were observed at m/z 332 and m/z 230, as shown in Figure 7; Figure 3 respectively. In addition, for the product (daughter) ions, TMT was observed at m/z 135 [M+H]+ and DMT at m/z 199 [M+H]+ as shown in Figure 8; Figure 4 respectively.
Method Validation
The validation process, conducted in accordance with (SWGTOX, 2013) guidelines, and was thoroughly evaluated by key performance parameters, including specificity, sensitivity, linearity, accuracy, recovery, and matrix effects. Matrix effects, defined as the influence of sample components other than the analytes on the quantification process, were carefully assessed to ensure precise and reliable measurements.
Specificity and Sensitivity
Excellent sensitivity and accuracy have been found for the detection of pyrethroid and organophosphorus pesticides in gastric fluid and urine samples by the rapid and highly specific method. With distinctive peak shapes and shorter retention times, the chromatographic performance was excellent, indicating that the method is very effective for frequent analysis of pyrethroid and organophosphorus pesticides. Clean separations were ensured by the chromatograms obtained using the SPE method, which identified the pesticide peaks. As shown in Figure 6; Figure 2, specificity tests were carried out at seven different concentrations and no interference peaks were found at the target retention times, indicating the high specificity of the method.
Linearity
The linearity of the method was evaluated by analyzing the drugs at seven different concentrations, utilizing linear regression analysis and analysis of variance. Calibration curves were constructed by plotting area response versus concentration (ng/mL), calculated using the slope-intercept equation. The resulting plots demonstrated strong linearity with an excellent fit, as indicated by the correlation coefficient (r²). The calibration curve for TMT and DMT was linear over the range of 2.5 ng/mL to 160 ng/mL, with r² values approaching 1, confirming the method's high accuracy and consistency as shown in Figure 11; Figure 10 respectively. The obtained values are summarized in Table 2.
Table 2
Linearity, LOD, LOQ, Intercept, and slope of Pyrethroid (TMT) and Organophosphorus (DMT).
LOD, LOQ and Recovery
A validation of the SPE extraction and cleanup method was carried out by assessing the Limit of Detection (LOD), Limit of Quantification (LOQ), and recovery. The limits of detection (LOD) for TMT and DMT were found to be 5.6 ng/mL and 3.08 ng/mL in gastric fluid, respectively, and 4.06 ng/mL and 5.31 ng/mL in urine. In gastric fluid, the LOQ varied from 9.33 ng/mL to 17.17 ng/mL, while in urine, it was between 12.31 ng/mL and 16.10 ng/mL (Table 2). Figure 13; Figure 12 shows the acceptable recovery rate of 70–120% was observed for both DMT and TMT. Remarkably, no earlier research has reported for the detection of TMT and DMT in simulated urine and simulated gastric fluid samples. As a result, this study led to the development of a robust UPLC-MS/MS method with a minimal total analysis time.
Matrix Effect and Interference
Two distinct concentration levels of matrix effects were applied to all analytes (lower quality control, LQC; 2.5 ng/mL; higher quality control, HQC; 160 ng/mL). For both quality control levels, we found variances of less than 20% from matrix effects for the analyte. These are acceptable findings that meet the validation requirements. The interference analysis demonstrated that the approach is specific for TMT and DMT because it failed to find a material that was similar to the simulated samples. TMT and DMT may be quantified using the currently suggested UPLC-MS/MS technique in a single run at a limit of detection of 2.5 ng/mL.
Conclusions
The ultrasonicator-aided cartridge-based extraction method for pesticide detection in simulated biological matrices via UPLC-MS/MS offers a promising solution to the challenges faced in pesticide analysis. By optimizing extraction conditions and employing advanced analytical techniques, this research aims to enhance the accuracy and reliability of pesticide detection, thereby supporting public health and environmental sustainability initiatives. A new method to quantify pyrethroid (TMT) and organophosphorus (DMT) pesticides in simulated gastric fluid and urine samples has been developed, integrating SPE, ultrasonicator-aided HLB cartridge-based extraction, and UPLC-MS/MS. Ultrasonic waves are used in the ultrasonicator to create cavitation, and collapsing microbubbles enhance sample agitation and extraction effectiveness. This technique shows its efficacy for precise analysis by allowing the consistent identification of TMT and DMT across seven concentration levels. To measure pyrethroid (TMT) and organophosphorus (DMT) in simulated stomach fluid and urine samples at seven different concentrations and is reliable for analyzing TMT and DMT. The developed method was precise and accurate in extracting the pesticides TMT and DMT, according to the validation of data from simulated gastric fluid and urine samples with a recovery rate of 70%-120% according to SWGTOX guidelines. The validation results demonstrated excellent linearity and accuracy, indicating that the method provided consistent and reliable responses across a wide concentration range. The LOD and LOQ were found to be satisfactory, highlighting the method's high sensitivity in detecting trace amounts of the pesticides TMT and DMT in simulated gastric fluid and urine samples. This sensitivity enables the detection of these compounds even at very low concentrations, which is crucial for both clinical and forensic applications. The optimized method underwent thorough validation, confirming its robustness in terms of linearity and trueness. The average relative recoveries were consistent with acceptable standards, demonstrating that the method yields accurate and reproducible results within a short analysis time. This aspect is particularly beneficial in scenarios where time is critical, such as in forensic investigations. Moreover, the versatility of the proposed protocol was evident. Not only was it effective for detecting pyrethroid TMT and organophosphorus DMT compounds, but it also holds potential for broader application. With appropriate adjustments, this method can be adapted for the detection of other pyrethroid and organophosphorus pesticides in biological matrices. This adaptability makes the protocol a valuable tool for clinical and forensic science laboratories, where reliable detection of pesticides in various samples is often required. The protocol’s applicability across different matrices further enhances its utility for toxicological analysis and forensic investigations.
Conflicts of interest
The authors declare that none of their known financial conflicts or interpersonal connections might have had an impact on the work outlined in this paper.
Author contributions
Pratik Singh: Investigation, Methodology, Data curation, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Arvind Kumar Maurya: Software, Data curation, Formal analysis, Visualization, Writing - review & editing. Ashish Singh Tanwar: Data curation, Formal analysis, Visualization, Writing - review & editing. Palak Sharma: Formal analysis, Visualization, Writing - review & editing. Majji Sai Sudha Rani: Formal analysis, Visualization, Writing - review & editing. Jasjeet Kaur: Conceptualization, Methodology, Formal analysis, Supervision, Writing - review & editing. Sanjeev Kanojiya: Conceptualization, Methodology, Data curation, Formal analysis, Supervision, Writing - review & editing. Prateek Pandya: Conceptualization, Methodology, Formal analysis, Supervision, Writing - review & editing.