INTRODUCTION
It is well known that the emergence of multiple resistances in clinically bacterial strains is a growing severe threat to human health. Resistance to currently conventional drugs presents an increasing global health threat concerning major clinical pathogens and currently antimicrobial agents (Levy & Marshall, 2004) . Bacterial infections with multidrug-resistant bacteria are hard to treat due to the absence of effective treatment, and in some cases, health care provides the necessity of using more toxic antibiotics or antifungal drugs for the patient like Candida infections. Because of these findings, there have been considerable efforts to develop new antimicrobial agents by screening natural products, enhancing existing antibiotics, and synthesizing new antimicrobial peptides. In this context, here we focused on using lichen as natural sources of new antimicrobial drugs since lichen compounds have different biological activities related to the species of lichen, type of solvent and microbial strains tested.
Lichen is a symbiotic organism composed of a mycobiont (a fungal species) and a photobiont algal species) with a stable and unique structure (Rascio & Rocca, 2013). Lichens have been used in industrial and medical fields (Money, 2016). They produce various chemical substances known as lichen acids. In addition, to other secondary metabolites owing important biological and pharmacological properties (Ranković, 2015). In particular, antimicrobial, antioxidant, anticancer, anti-inflammatory, analgesic and antipyretic potentialities were described for lichens. The antimicrobial efficacy of numerous lichen genus has been reported in the literature, for example Cladonia, , Parmelia, Peltigera, etc. (Ranković, 2015) .
In addition, among the multiple secondary metabolites synthesized by Cladonia species, we appointed atranorin, hypoprotocetraric acid, fumarprotocetraric acid and usnic acid (Kosanić, Ristić, Stanojković, Manojlović, & Ranković, 2018). We have recently described the superior antimicrobial activities of some lichen species in Tunisia (Mendili, Essghaier, Seaward, & Khadhri, 2021) . Thus, this work presents a continuation of our previous research, so that we aim to research other species of lichens located in Tunisia (Cladonia species). No previous reports have demonstrated the activity of Quencher extracts from these species, as well as methanolic or acetone extracts. Therefore, this study pioneered to elucidate the anti-microbial activities of methanolic, acetone and quencher lichen extracts from Cladonia rangiformis and Cladonia pocillum located in Tunisia.
MATERIALS AND METHODS
Lichen sampling
Cladonia rangiformis Hoffm. and Cladonia pocillum Ach. were obtained in February 2016 in Tunisia's Bazina region (36°.95′05.80N, 09°29′73.84′′E). Voucher specimens have been placed in Tunisia's Faculty of Sciences, Department of Biology's Lichenological Herbarium.
Extraction with organic solvents
Cladonia species (20 g each) were dried at room temperature in the dark, and chemicals extracted using acetone and methanol (200 mL). At room temperature, the ultrasonic extraction was carried out for two hours. Filtered extracts were concentrated on a rotary evaporator. The crude extracts were stored at a temperature of +4 °C until analysis.
QUENCHER approach extraction
The QUENCHER approach present a new, quick, simple, cheap and reproducible method used to quantifying phenolic compounds and measuring total antioxidant activity (Gökmen, Serpen, & Fogliano, 2009) . In this study, we have used this method to elucidate the antimicrobial activity of Cladonia species as previously described by (Mendili et al., 2021) .
Antimicrobial potentialities evaluation
Microorganisms
In order to evaluate the antimicrobial activities of the Cladonia rangiformis and Cladonia pocillum extracts, a list of bacteria and fungi human clinical strains were used as following: Enterobacter cloacae; Escherchia coli, Staphylococcua aueurs, Enterococcus faecalis , Candida albicans, C sake; C parapsilosis . Penicillium digitattum; Aspergillus niger Alternaria alternata. Cultures were prepared as previously described by (Mendili et al., 2021).
Agar diffusion method
The agar diffusion method was used as recently described by (Essghaier, Dridi, Arouri, & Zid, 2021). Prior to use, each lichen extract was diluted with sterile water and the concentration was adjusted to 2000 µg/mL. To sterilise the extract solution, it was filtered through a 0.22 m pore size filter. The pathogen concentration was increased to 107 CFU/mL for bacterial strains and 105 spores/mL for fungal strains, and 100 µL of each concentration was applied individually to the surface of the appropriate agar plates (the Mueller-Hinton media and the potato dextrose agar were used for the antibacterial and the antifungal assays, respectively).
A clear zone surrounding the well indicates the presence of activity against the pathogen under investigation, and the diameter of the inhibition zone was measured in millimetres. Three times were conducted per exam. Ceftazidime CAZ30 and the fungicide Voriconazole VCZ were used as standard.
Minimum Inhibitory Concentration (MIC determination
The MIC values given in µg/mL were determined using the microdilution broth method in 96 well flat microliter plates.The MIC value, which denotes the lowest antimicrobial inhibition concentration, was visually determined by the absence of turbidity in the well, with three independent replicates (Thakur, Barua, & Karak, 2015).
Bactericidal and fungicidal activity determination
The previous method described by (Graciela et al., 1995) was used to determine the bactericide activity of tested extracts expressed in arbitrary units per ml (AU/mL). We have transferred 50 μL onto an agar culture medium surface previously inoculated with 105 CFU/mL of the tested pathogen from a serial twofold dilution of the Cladonia extracts. It was determined that AU/mL was equal to 1000X D/A (where A is the volume of the extract spotted on agar (50 μL in this example) and D is the reciprocal of the greatest dilution providing an inhibition zone for the indicator strain) (Mendili et al., 2021).
Lysozyme activity
To demonstrate the lysozyme activity of each lichen methanolic extract against gram-positive bacteria cells by measuring the absorbance at 660 nm of gram positive cell bacteria. we have applied the turbidimetrically method of (Ryazanova, Stepnaya, Suzina, & Kulaev, 2005) as detailed in (Sehimi, Essghaier, Barea, Sadfi-Zouaoui, & Zid, 2019) .
Antifungal activity
Spore germination inhibition
The conidial suspensions from each tested fungi (Penicillium sp., Aspergillus sp.) were prepared as recently detailed by (Mendili et al., 2021). An incubation at 21°C for 24 h was followed by the addition of the conidial suspensions, the tested extract, and 1 mL of 5 percent glucose to the mixture. Microscopic inspection of each extract (E) using the (Sarangi, Athukorala, Fernando, Rashid, & Kievit, 2010) method yielded the percentage of extracts that inhibited spore germination (I percent).
Mycelial hyphae destruction and microscopic observation
The effect of each lichen extract was tested on the mycelial hyphae morphology of Alternaria pathogen. The preparation of hyphae mycelial suspension was done as recently reported by (Mendili et al., 2021). Briefly, a mycelial solution expressed in mg/mL was prepared in Tris-HCl buffer (0.01 M, pH 8) after three lavages of fungal culture with sterile water and recuperation of mycelial pellet centrifugation at 8000 rpm for 10 min. In an Eppendorf tube, we added 500 μL of 5000 μg/mL of the methanol extracts to 500 μL of mycelial hyphae solution and incubated at 37°C for 14 h. Optical density (OD) was measured at 540 nm. An increase of OD compared to the control tube (containing only a mycelial suspension) suggested the destruction of fungal hyphae by the presence of the methanol extracts (Ryazanova et al., 2005) .
Results
Detection of antibacterial and anti-Candida activities
The antimicrobial activities of the methanol, acetone as well as QUENCHER extracts of Cladonia pocillum and Cladonia rangiformis were analyzed against the pathogens: Enterobacter cloacae; Escherichia coli, Staphylococcus aureus, Enterococcus faecalis and three yeasts species (Candida albicans, C. sake and C. parapsilosis). The results obtained revealed that the methanolic extract of C. pocillum provided the highest diameter of the inhibitory zone with 31 mm and 27.5 mm against E. cloacae and E. coli, respectively (Figure 1).
Figure 1
Observation of zone inhibition onagar plate obtained by the acetone, quencher and methanolic extracts of both Cladonia species.
a: Enterobacter cloaceae, b: Escherichia coli and c: Enterococcus faecalis and S present the corresponding organic solvent used for acetone or methanol extraction and quencher extract the distilled water was used as a solvent.
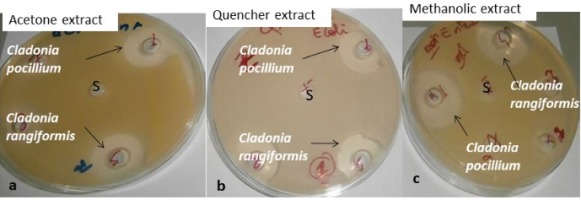
For the C. rangiformis, a higher inhibition diameter (22.5 mm) was observed with the methanol and quencher extracts. Based on this finding, the methanol extract from C. pocillum was the most effective against all tested gram-positive and gram-negative bacteria, compared to C. rangiformis extracts (Table 1).
Table 1
Antimicrobial activity of Cladonia rangiformis and Cladonia pocillum extracts evaluated against pathogen strains species tested at 2000 µg/mL, as detected in the agar well diffusion test, values are expressed in mm
The methanol and acetone extract of C. rangiformis exhibited high inhibition diameters against all tested yeast Candida species for anti-yeast activity. The methanol extract of C. rangiformis showed high activity against C. parapsilosis, with an 18.5 mm inhibition zone. Methanolic and quencher extracts of C. pocillum were only active against Candida albicans, followed by the acetone extract against Candida parapsilosis.
In addition, only methanolic extract of C. pocillum was more effective against E. coli, E. cloaceae and Candida albicans than the Ceftazidime CAZ30 and the fungicide Voriconazole VCZ, respectively (Table 1).
Table 2 shows the variations in MIC (minimum inhibitory concentration) values between the different extracts utilized. MIC values ranged from 250 μg/mL to 2000 μg/mL. Methanol extracts of C. pocillum were more active against E. cloacae at only 250 μg/mL. The methanol and acetone extracts of C. rangiformis were active against S. aureus at only 500 μg/mL. However, the quencher extracts of C. rangiformis and C. pocillum could inhibit all bacterial species at the same MIC value (2000 µg/mL), unlike C. pocillum was more active against E. faecalis at 500 µg/mL. The acetone and methanolic extracts of C. rangiformis have the best MIC against Candida spp. studied. It is noted that the quencher extracts of C. pocillum inhibited Candida albicans (500 μg/mL) (Table 2).
Table 2
MIC of the extracts of C. rangiformis and C. pocillum against the pathogen strains. Values were expressed in ug/mL
Bactericide and lysozyme activities
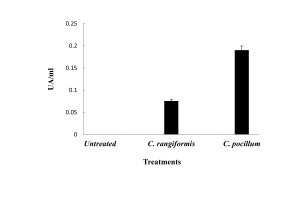
In order to evaluate the antibacterial potential of methanol extract of C. rangiformis and C. pocillum, other parameters were determined as lysozyme effect. The lysozyme activity was tested against two tested gram-positive bacteria species, E. faecalis and S. aureus (Figure 2), and the bactericide activity expressed in UA/mL (Table 3). As a result, C. pocillum has a superior lysozyme potential against S. aureus, with 160 AU/mL, and against E. faecalis, with 80 AU/mL values. In contrast, C. rangiformis have a lower lysozyme potential against S. aureus, not exceeding 15AU/mL, also being not effective against E. faecalis (Figure 2).
Figure 2
Lysozyme activity effect of both methanolicextracts from Cladonia rangiformis and Cladonia pocillum species. Error bars represent the SE of themeans.
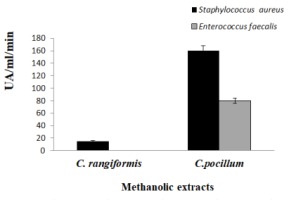
Table 3
Bactericidal and fungicidal activities of the Cladonia extracts expressed in UA/mL
Table 3 Illustrates the bactericide activity and the methanolic extract of C. pocillum, owing to the highest efficacy against E. cloaceae with 160 UA/mL, following by methanol and acetone extract of C. rangiformis and quencher extract of C. pocillum against S. aureus and E. feacalis, with 80 UA/mL, respectively.
Antifungal activity
The probably inhibitory activity of Methanol , acetone and Quencher extracts obtained from both Cladonia species (C. rangiformis ; C. pocillium) on the spore germination of the test filamentous fungi Aspergillus niger and Penicillium digitatum were investigated in Figure 3.
Figure 3
Effect of Various extracts produced from Cladonia rangiformis and Cladonia pocillum on spore germination of Penicillium (A, B) and Aspergillus (C, D) Values show the spore germination in percentage
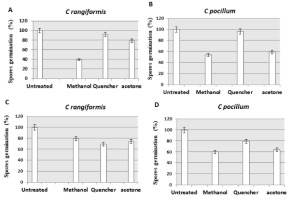
The results show that the methanolic extract had a high negatively effect on the germination of spores. The methanolic extract of C. rangiformis had 60.7% inhibition against Penicillium spores. The methanolic extract of C. pocillum had 46.4% and 41% inhibition of Penicillium and Aspergillus spores, respectively. In turn, C. rangiformis specie (Quencher extract) mentioned a less effect on the germination of Aspergillus spores with a percentage inhibition of 30.7% (Figure 3).
Furthermore, the methanolic extracts of C. rangiformis and C. pocillum were able to destroy Alternaria hyphae so that the methanolic extract of C. pocillum presented 2.7 times more destruction than the methanolic extract of C. rangiformis (Figure 4).
Discussion
At present, owing to the infection’s pathogens and the opportunists' microorganisms that treat human health, the uncontrolled use of antibiotics and the increasing of the multi-resistance mechanisms, great attention was directed to the discovery of a safe alternative approach to harmful synthetic drugs. Thus, Lichens are natural sources for therapeutic compounds by producing secondary metabolites exhibiting biological potentialities like antimicrobial activity (Barnes, 2000; Plaza et al., 2017). Numerous publications have revealed the biological actions of crude extracts and purified lichen components, including antioxidant, antibacterial, antiviral, cytotoxic, insecticidal, and anti-inflammatory characteristics (Mendili, Bannour, Araújo, Seaward, & Khadhri, 2021; Mendili et al., 2021; Ranković, 2015). In this context, the Cladonia genus was previously described as secondary metabolites source (Kosanić et al., 2018). Thus, this was the first study in Tunisia to examine the antibacterial and antifungal properties of methanolic, acetone, and quencher extracts of C. rangiformis and C. pocillum species. They have exhibited antibacterial and anti-Candida effects, as well as an inhibiting spore germination of filamentous fungi. Therefore, the antimicrobial potential depend on the species, the nature of the extract, and the extraction methods. As a result, the differences are species-specific, extract-specific, and extraction-method-specific. It was noted by (Mendili et al., 2021) that the antimicrobial activity was influenced by the extraction method as well as the type of solvent and confirmed by Plaza et al. (2017). Our results corroborate other studies indicating that Cladonia species exhibiting high antimicrobial activity against bacteria species belonging to gram positive and gram negative groups (Açıkgöza et al., 2013; Kosanić et al., 2018).
In this work, we described the behavior of the powdered material without organic solvent (Gökmen et al., 2009) as a new method used firstly for the measurement of antifungal and antibacterial behavior of extracts obtained from lichen species C. pocillum and C. rangiformis. As a result, they showed a high antibacterial action against gram-positive and gram-negative bacteria and antifungal activity against Candida and other species. The methanolic extracts of C. rangiformis and C. pocillum were more active against C. albicans, C. parapsilosis and C. sake, with the most significant zone inhibition exceeded 13mm. However, Plaza et al. (2017) indicated that Cladonia affrappii extracts showed antifungal effect against C. albicans at 20 mg/mL, with zone inhibition not exceed 11.9 mm. In the present study, we have described a successful extraction method since our various extracts were able to give the most potent antibacterial and antifungal effects compared to other lichen species extracts reported by comparing the MIC values varied from 0.25mg/mL and 2mg/mL concerning bacteria and fungi species. On the contrary, MIC values obtained by the acetone, the methanolic and the aqueous extracts from Leacanora atra, Parmelia saxatilis and Parmeliopsis ambigua speciesvaried from1.56 to 12 mg/mL for bacteria and from 12.5 to 25 g/mL concerning tested fungal species (Ranković & Kosanić, 2012).
The efficiency of the antimicrobial potential of our C. rangiformis and C. pocillum various extracts was confirmed by their MIC values, which ranged from 0.25 mg/mL to 2 mg/mL so that these findings were more important than those reported by Cladonia affrappii extracts against Candida species which varied from 2.2 mg/mL to 11.9mg/mL (Plaza et al., 2017).
In contrast to previous studies describing the moderate antifungal potential of Cladonia species (Ranković, Ranković, & Marić, 2010; Verma, Behera, & Parizadeh, 2011), here we mentioned the most potent antifungal effect these described Cladonia species. Moreover, the acetone extract of C. rangiformis possesses an inhibitory effect against C. albicans, C. parapsilosis and C. sake, which is opposite to the results described by Verma et al. (2011) who found no activity against C. albicans neither by acetone and or methanol extracts from C. ochrochrola.
Our study investigated the antimicrobial, lysozyme and antifungal effects of various extracts of C. rangiformis and C.pocillum species. Recently (Mendili et al., 2021) mentioned that the similar extraction method by methanol, acetone and quencher from four lichens: Diploschistes ocellatus, Flavoparmelia caperata, Squamarina cartilaginea, as well as Xanthoria parietina, have antibacterial, lysozyme, in addition to antifungal properties.
Ersoz et al. (2017) revealed that the extract of C. pocillum had cytotoxic, anti-proliferative, antioxidant, apoptotic, and antimicrobial activities. In addition, (Mendili et al., 2021) described the antimicrobial efficacy of some Tunisian lichen species and the antioxidant properties and phenolic compounds produced by the species C. rangiformis. They also showed a positive correlation between antioxidant activity and phenolic compounds (Mendili et al., 2021) , which could prove that the antimicrobial capacity of lichens was positively correlated to their phenolic content. Moreover,Yucel, Odabasoglu, Gullue, Çalik, and Çakir (2007) suggested a relation between antioxidant and antimicrobial activities of the chloroform extract from C. rangiformis. In addition, several works have evaluated a positive correlation between phenolic constituents of lichen and their antimicrobial activity (Buçukoglu, Albayrak, & Halici, 2012; Gulluce, Aslan, Sokmen, Sahin, & Adiguzel, 2006; Kosanić et al., 2018; Rankovic, Misˇic, & Sukdolak, 2007) .
Conclusion
The antimicrobial activity of ground material of C. rangiformis and C. pocillum showed an inhibitory effect against clinical bacterial pathogens and antifungal activities against Candida and filamentous species. According to these results, the chemical compounds related to the antibacterial and antifungal effects should be identified.