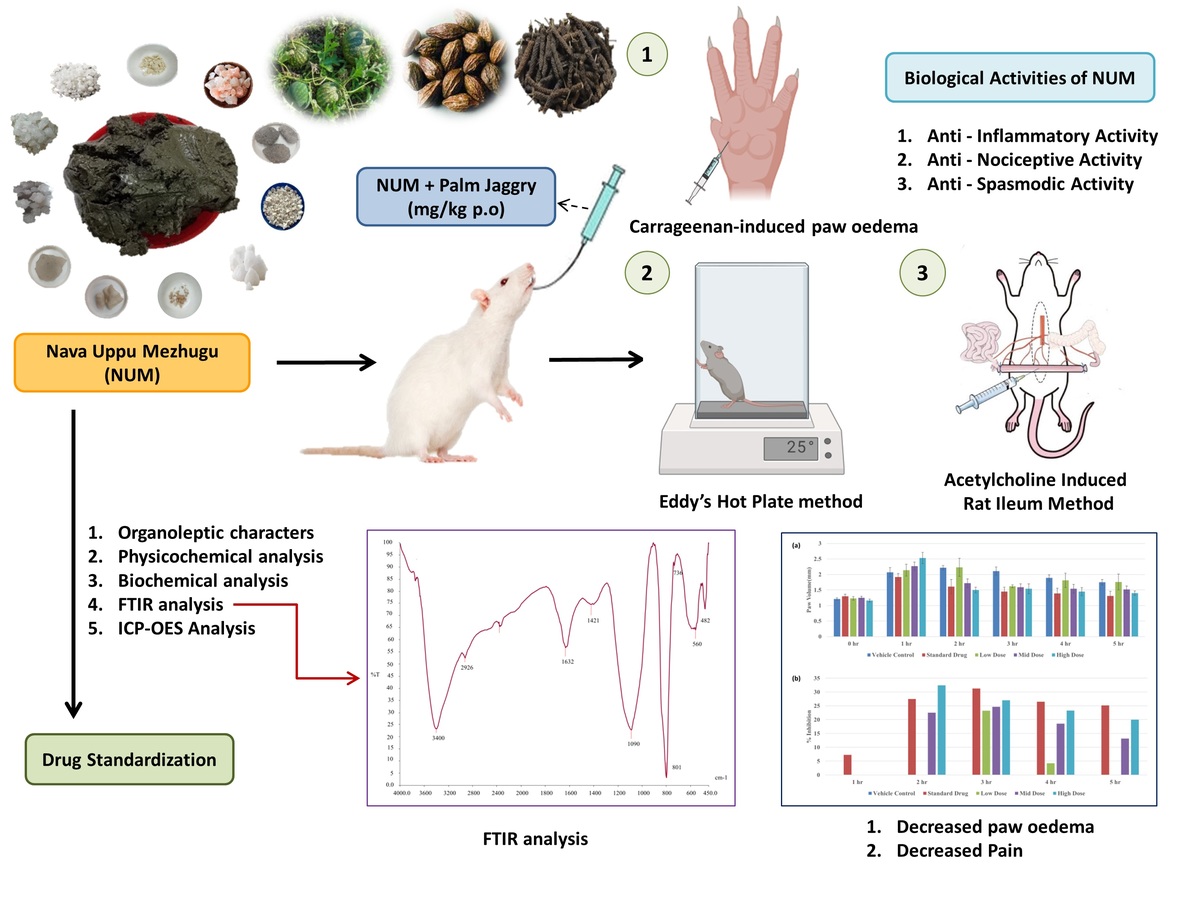
INTRODUCTION
Rheumatoid arthritis (RA) is characterized by symmetrical swelling of smaller joints at the initial stage that progresses to larger joints and this autoimmune condition potentially impacts various organs, such as the skin, eyes, heart, kidneys and lungs. It frequently leads to joint damage, such as bone and cartilage erosion, along with weakened tendons and ligaments (Bullock, Rizvi, & Saleh, 2018; Lee, Kim, Cho, & Lee, 2017). The etiology of RA involves a significant genetic component, including interactions between genotypes and environmental factors (Chauhan, Jandu, Brent, & Ma, 2023). Epigenetic changes (Weyand, Hicok, Conn, & Goronzy, 1992; Wu, Liao, & Li, 2018), environmental factors such as cigarette smoking and silica exposure (Stolt, Bengtsson, & Nordmark, 2003) and alterations in the composition of the gut microbiome (Espina et al., 2019) are associated with increased RA risk. Genetic factors contribute 40-65% of RA seropositive individuals and approximately 20% of RA seronegative individuals (Kłodziński & Wisłowska, 2018).
RA is more common in women than in men and occurs three times more frequently in women than in men. It typically emerges between the ages of 30 and 50 years but can occur at any age (Venetsanopoulou, Alamanos, Voulgari, & Drosos, 2023). The prevalence of RA varies globally from 0.24% to 1%, with higher rates in developed countries such as the USA and Europe (Alamanos, Voulgari, & Drosos, 2006; Safiri, Kolahi, & Hoy, 2019). The incidence trends differ by region, with increases observed in some areas, such as Africa (Adebajo, 1991). In India, the prevalence of RA is 0.7%, which is greater than the prevalence rate of 0.46% in all over world (Almutairi, Nossent, Preen, Keen, & Inderjeeth, 2021; Bagepally, Kumar, Sasidharan, Haridoss, & Venkataraman, 2023; Malaviya, Kapoor, Singh, Kumar, & Pande, 1993). RA patients have higher mortality rates (up to 1.5–1.6 times) than the general population, primarily due to cardiovascular, respiratory, and infectious diseases (Hoek, Boshuizen, & Roorda, 2017; Venetsanopoulou et al., 2023).
The existing treatments for RA, such as “nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids (GCs) and disease-modifying antirheumatic drugs (DMARDs)”, greatly manage symptoms and disease progression. However, these treatments are accompanied by gastrointestinal and cardiovascular complications and immunosuppression (Buttgereit, Silva, & Boers, 2002; Geraldino-Pardilla & Bathow, 2015; Negrei, Bojinca, & Balanescu, 2016). Consequently, there is a growing demand for alternative approaches that can effectively treat RA with fewer adverse reactions.
In the Siddha system of medicine, diseases are categorized into 4448 types based on the affected vital humors, organs, and systems (Siva, 2022). Saint Yugi further classified the various neurological skeletal diseases among 80 types of Vatha diseases in his text Yugi vaithiya cinthamani. Uthira Vatha Suronitham (UVS) is one of these eighty types of vatha diseases. The clinical manifestations of UVS may be clinically correlated with the symptoms of RA. UVSs are also known as Muṭakku vātam and Valiazhal Kiil vaayu in the Siddha literature (Muthaliyar & N, 2016; Siva, 2023).
Traditional Indian medicine, particularly Siddha medicine, is a rich source of potential treatments for RA. Siddha medicine is based on ancient principles and utilizes a wide range of herbs, minerals and other natural substances to address various ailments, including inflammatory conditions such as RA (Arumugam, Gunalan, Firdouse, & Arivazhagan, 2023). The Siddha system has 32 internal medicines that have a certain shelf life and extensively uses purified metals, minerals and inorganic substances. Mezhugu, a viscous semisolid preparation from one of the abovementioned 32 internal medicines, has a shelf life of 5 years. It is prepared via two methods: grinding and heating (Thiyagarajan, 2009).
Nava uppu mezhugu (NUM) is a herbo-mineral drug that is prepared from 9 inorganic salts, 2 pashanams and other herbal raw ingredients by grinding. It is indicated for Cūlai (Throbbing pain), Muṭakku vātam (Rheumatoid Arthritis), Cūtaka vāyu (Dysmenorrhoea, PCOD, Amenorrhoea), Makōtaram (Ascities), Kiranti (Lymphadenities, chronic skin ulcers), Mēkam (Veneral disease), Nīrkkōvai (Rhinitis), Utara vāyu (Flatulance), Viṣavātam (Virulance), Aṇṭavātam (Hydrocele), Pārica vāyu (Hemiplegia) and Curam (Fever). This drug works well in treating diseases that cause pain and inflammation, such as vaatha-type diseases (Mudhaliyar & N, 1998; Muthaliyar & N, 2016).
Exploring the potential of Siddha medicine for the management of RA could provide valuable insights into novel therapeutic approaches that are safe, effective and potentially free from the adverse effects associated with conventional treatments. Combining the wisdom of traditional medicine with modern scientific research may reveal new treatment options that offer hope for RA patients seeking relief from their symptoms and maintaining their quality of life. NUM is safely used for pain and inflammatory conditions by siddha medical practitioners, but its therapeutic efficacy has not been properly evaluated or documented. Hence, the aim of the present study was to assess the anti-inflammatory, anti-nociceptive and anti-spasmodic activities of Nava uppu mezhugu in various animal models.
MATERIALS AND METHODS
Ingredients of Nava Uppu Mezhugu
The ingredients of NUM are listed in Table 1.
Table 1
Ingredients of Nava Uppu Mezhugu.
Procurement of the raw drugs and drug ingredients
All the raw drugs and ingredients were procured from the Authenticated Country Raw Drug Store in Chennai. Cow’s milk was collected from a nearby farm around the Tambaram.
Authentication of the raw drugs
Mineral drugs and milk products were identified and authenticated by the Head of the Department of Gunapadam at the National Institute of Siddha, Chennai. The raw plant drugs were identified and authenticated by a botanist at the same institute. The authentication numbers are NISMB5882023 and Gun/Aut/025A/22, dated on 25.11.2022.
Preparation of Nava Uppu Mezhugu
In the initial step, all the ingredients were purified as described in the Siddha literatures (Murukesa & Su, 2013; Sornamariyammal, 2022; Thiyagarajan, 2009). The salts listed in Group A were finely ground and placed in an earthen pot. To this mixture, Tumaṭṭi cāru and cow's milk were added. The combined ingredients were then filtered and boiled. The resulting mixture was thoroughly blended with the root of Tutti vēr until it achieved a semisolid (kulampu) consistency. After allowing the mixture to cool, it was ground into a powder. The drugs in Group B were also ground into a fine powder. This powder was then combined with the previously prepared mixture. The combined powders were triturated with Tiripalai kuṭinīr for 3 hours. After this initial trituration, cow's milk was added, and the mixture was triturated again for an additional 3 hours until it reached a waxy (Meluku) consistency (Figure 1). Finally, the prepared NUM was stored appropriately in an airtight container (Mudhaliyar & N, 1998).
Quality control and standardization procedures for NUM
An analytical study of NUM validated the drug for medicinal use through comprehensive quality and efficacy assessments. These analyses were conducted as per the Pharmacopoeia Laboratory of Indian Medicine (PLIM) protocols, including organoleptic, physicochemical, and biochemical evaluations, as well as active principle and element analyses. The organoleptic characteristics assessed were color, odor, and taste. Physicochemical analyses included measuring the moisture content (loss on drying), pH, total ash content, acid-insoluble ash content, water-soluble extractive content, and alcohol-soluble extractive content (Annamalai, Srinivasan, & Thavaseelan, 2024; Lohar & Government of India, Department of AYUSH, Ministry of Health & Family Welfare, Pharmacopoeial Laboratory for Indian Medicines., 2008). Biochemical analysis (Anonymous, 2005; Anonymous, 2009) and instrumental techniques such as Fourier transform infrared (FTIR) spectroscopy and inductively coupled plasma‒optical emission spectrometry (ICP‒OES) were studied (Niraimathi, Lavanya, & Brindha, 2022; Sharma, 2020).
Anti-Inflammatory Activity of NUM in Wistar Albino Rats
Selection of experimental animals
This study employed healthy Wistar albino rats of both sexes weighing 140-160 grams obtained from the laboratory. All participants were subjected to rigorous monitoring for infection, and any rats exhibiting symptoms of the condition were excluded from participation in the study. Compliance with animal welfare legislation was observed throughout the animal experiment.
Preparation of experimental animals
The rats were randomly assigned to five groups, each containing six animals, and were treated as given in Table 2.
Table 2
Selection of Experimental Animals for anti-inflammatory activity of NUM in Wistar Albino rats.
Carrageenan-induced rat paw edema method
The anti-inflammatory effects of NUM were assessed in Wistar albino rats using the carrageenan-induced rat paw edema model as outlined by Winter et al., 1963. The rats were fasted overnight, and the test drug was administered orally via gastric gavage tubes and suspended in a vehicle. One hour post administration of the test drugs, all groups received a sub plantar injection of 0.1 ml of 1% carrageenan in the right hind paw. Paw thickness was measured using a plethysmograph at baseline and at hourly intervals for up to 6 hours following carrageenan injection. The percentage inhibition of paw thickness in the treated groups was determined by comparing their mean paw thickness to that of the control group (Winter & Risely, 1963).
% Inhibition = 100 (1-Vt/Vc)
(V - Control mean paw thickness, Vt - Test mean paw thickness).
Evaluation of Anti-Nociceptive Activity of NUM in Swiss Albino Mice
Selection of Experimental Animals
This study employed healthy Swiss albino mice of both sexes, weighing 25–40 grams, which were obtained from the laboratory. All participants were subjected to rigorous monitoring for infection, and any mice exhibiting symptoms of the condition were excluded from participation in the study. Compliance with animal welfare legislation was observed throughout the animal experiment.
Preparation of experimental animals
Swiss albino mice were randomly assigned to five groups, each consisting of six animals, and were treated as given in Table 3:
Table 3
Selection of Experimental Animals for anti-inflammatory activity of NUM in Swiss Albino mice.
Eddy’s hot plate method
The hot plate test was employed to measure response latency time following the procedure described by (Eddy & Leimbach, 1953). Mice that exhibited an initial nociceptive response within 15 seconds were selected for the study. The cut-off time for hot plate latencies was set at 15 seconds. The overnight-fasted mice were given the test drugs orally via gastric gavage tubes, with the drugs suspended in a vehicle. The standard drug was administered intraperitoneally. The animals were placed on a hot metal plate maintained at 55°C and surrounded by a Plexiglas cylinder (26 cm in height, 19 cm in diameter). The time elapsed between placing the animal on the hot plate and the occurrence of hind paw licking, shaking, or jumping off the surface was recorded as the response latency in seconds. The specificity and sensitivity of the test were enhanced by recording the reaction time of the first observed behavior, whether it was paw licking or jumping. Responses were measured at 0, 60, 90, and 120 minutes (Eddy & Leimbach, 1953).
The percentage protection against thermal pain stimulus was calculated using the following formula:
% Protection = Test Mean – Control Mean/Test Mean X 100
Evaluation of the Anti-Spasmodic Activity of NUM in the Excised Rat Ileum
Selection of Experimental Animals
The animal source for the study was Kerala Veterinary and Animal Science University, Kerala, Mannuthy, utilizing Wistar albino rats (one male) aged 6-8 weeks with a body weight of 200-220 grams. The animals were acclimatized for 7 days prior to dosing, with veterinary examinations conducted at the time of purchase and at the end of the acclimatization period. The housing conditions included a temperature of 24 ± 2°C, relative humidity between 30% and 70%, and a 12:12-hour dark and light cycle. Each animal was identified by individual marking, animal number, and cage number using picric acid. The diet consisted of rat chow pellets and RO water, and the animals were housed in stainless steel cages with dry grass bedding.
Acetylcholine-induced ileum formation in rats
Overnight-fasted adult male Wistar albino rats weighing between 200 and 220 g were used in this study following the methods outlined by (Carvalho, Almeida, Melo, Cavalcanti, & Marçal, 2009). Under mild pentobarbital anaesthesia, the rats were euthanized by cervical dislocation. The abdomen was opened, and two pieces (each 2 cm long) of the ileum were dissected from a segment 10-15 cm proximal to the ileocecal junction. Both isolated ileum segments were mounted for tension recording and allowed to equilibrate for 1 hour in separate 50 ml organ baths containing aerated normal Tyrode solution (composition in mM: NaCl, KCl, CaCl2, MgCl2). H2O, NaHCO3, NaH2PO4, and glucose; pH 7.4). The tissues were maintained at 37°C and bubbled with air.
The response of the first ileum was recorded using a frontal lever with various concentrations of NUM (ranging from 0.12 µg to 32 µg). The response of the second ileum was recorded using a frontal lever with various concentrations of acetylcholine until submaximal responses were obtained. The isolated ileum was then incubated for 45 minutes with NUM (1. 2 µg/ml). Following this incubation period, the response of the ileum to various concentrations of acetylcholine was recorded again in the presence of NUM (Carvalho et al., 2009).
Statistical analysis
The observed results were statistically analysed using one-way ANOVA followed by Dunnett’s test. A p value less than 0.05 was considered to indicate statistical significance. The study drug NUM showed statistically significant effects (*P < 0.05, **P < 0.01, ***P < 0.001) compared to the standard drug, with values expressed as the mean ± SD.
RESULTS AND DISCUSSION
Currently, Siddha medicines are more popular and mostly desired by people in need. However, the lack of scientific validation is a major disadvantage. Therefore, all siddha medicines must be subjected to pharmacological activity evaluation and clinical trials after standardization and instrumental analysis. To date, all related research on siddha has been performed based only on traditional literature and practices. These studies were performed on the basis of reverse pharmacology. The test drug NUM was prepared according to the siddha classical literature, and its organoleptic characteristics were subsequently validated. Physicochemical parameters and instrumental analysis were subsequently performed to determine its desirable pharmacological profile.
Organoleptic characters of Nava Uppu Mezhugu
The organoleptic characteristics of the NUM were determined, and the results are given in Table 4. These characteristics revealed that NUM was gray in color, wax-like semisolid, sour and bitter in taste and aromatic, resembling the characteristics of camphor milk and salt. These characteristics prove the epic nature of Mezhugu according to the Siddha literature.
Physicochemical analysis of Nava Uppu Mezhugu
The results of the physicochemical analysis of NUM are tabulated in Table 5. The total ash, water soluble ash and acid insoluble ash contents were 10.00 ± 0.50%, 8.50 ± 0.050% and 7.00 ± 0.020%, respectively. The total ash value indicates the drug purity, contamination, cleanliness of preparation, adulteration and substitution. Very low amounts of Ash are desirable, and the total ash content determines the presence of inorganic compounds within this range. The water soluble ash content was within the normal range, at 8.50 ± 0.050%, due to the presence of inorganic salts, and the acid insoluble ash content, at 7.00 ± 0.020%, was due to the presence of inorganic compounds such as silica and mercurial compounds. The water soluble and acid insoluble values indicate the amount of the drug completely dissolved in water or acid.
The extraction value indicates the concentration of active components in the sample when extracted using a solvent medium, such as water or alcohol. The water-soluble and alcohol-soluble extract values indicated the proportion of polar and nonpolar compounds in NUM. The extract value in alcohol was 7.90 ± 0.210, and that in water was 8.90 ± 0.510. This shows that the test drug NUM is more water soluble than alcohol due to the presence of more polar soluble components in the test drug. The loss on drying test is performed to calculate the amount of water and volatile matter present in the drug. The moisture of the drug was also calculated to evaluate the water content present in the drug. The loss on drying and moisture of the NUM was calculated to be 1.20 ± 0.540 and 8.90 ± 0.120%, respectively, which are within the normal range. This determines the shelf life, stability and quality of the drug. The pH of NUM is 8.30, and NUM is slightly alkaline in nature; the pH determines its role in digestion, absorption and bioavailability. Therefore, it is expected that the drug will be absorbed rapidly in the stomach when taken orally. This suggests that NUM has better bioavailability.
Table 5
Results of the physicochemical analysis of NUM.
Table 6
Results of FTIR analysis of NUM.
Table 7
Results of ICP‒OES analysis of NUM.
Figure 3
(a) Anti-inflammatory activity of NUM in Wistar albino rats; (b) Percentage inhibition of the anti-inflammatory activity of NUM.

Biochemical analysis of Nava Uppu Mezhugu
According to the results of the biochemical analysis, only sulphate and carbonate were present in the acid radicals, whereas only zinc, calcium, ammonium, potassium, and sodium were present in the basic radicals. In various tests, starch and reducing sugars were found, and other radicals were not present in the test drug sample. These values lie under the normal desired therapeutic value.
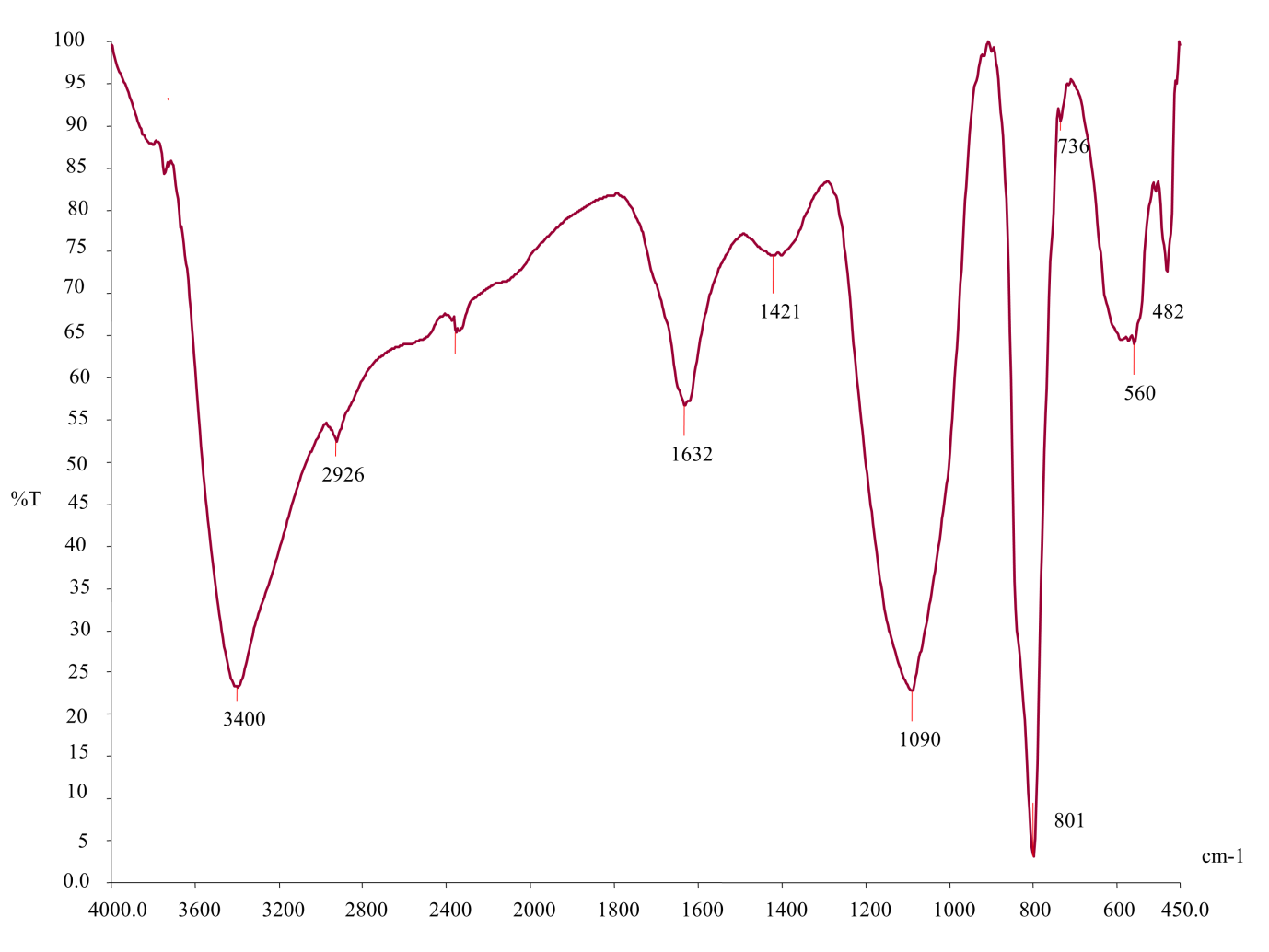
Fourier Transform Infrared Spectroscopy (FTIR analysis of Nava Uppu Mezhugu)
NUM exhibited the highest concentrations of O-H (3400), C-H (2926), C-O (1632), C-Br (1421), C-I (1090), C-E (801), C-736 (560), C-482 (482) and C-24 (2400) in the FTIR spectra. These results show the presence of certain organic functional groups, including alcohol (Al), methylene (amide), methylene (ethylene), secondary alcohol (secondary alcohol), halo compounds (Halo), and sodium (Na) (Figure 2,Table 6).
Inductively Coupled Plasma‒Optical Emission Spectrometry (ICP‒OES Analysis)
The results of the ICPOES analysis of NUM revealed that the concentrations of heavy metals such as arsenic, cadmium, copper, manganese, and lead were significantly lower than the detection levels. The results are tabulated in Table 7. Mercury (03.235 mg/L) was also found to be within the permissible levels in the sample. Mercury perchloride, a naturally occurring mineral of mercury, is one of the primary components of NUM in combination with Sulphur. Consequently, the concentration of Sulphur in the sample is greater than that of mercury. Furthermore, the analysis revealed a high presence of essential minerals such as calcium, sodium, potassium, phosphorus, zinc, magnesium, and sulphur because salts are the main ingredients in the test drug. This proves the safety of NUM for human consumption.
Biological Activities of NUM
Anti-Inflammatory Activity
The carrageenan-induced paw oedema in Wistar albino rats is the most commonly used method for performing acute anti-inflammatory screening pharmacology studies. Following the initiation of inflammation in the rat paw, the inflammatory process begins and continues. The first phase (0-2 hours) of inflammation is mediated primarily by histamine and serotonin, as well as an increase in prostaglandin production. The second phase of inflammation (2-3 hours in duration) is sustained by bradykinin-mediated release and the release of leukotriene and polymorph nuclear cells. NUM was administered orally at 6, 12, and 24 mg/kg at low, middle, and high doses, respectively. No inhibition of edema was observed in the low-dose groups at 6 hours. In the moderate-dose and high-dose groups, moderately significant changes were noted. In the mid-dose group, at 2 hours after test drug administration, moderately significant changes in edema reduction were noted but were less pronounced than those in the high-dose group. At 2 hours after drug administration, more significant changes were noted in the high-dose group than in the standard drug group, which was treated with 5 mg/kg indomethacin. The highest rate of inhibition was observed in the high-dose group (24 mg/kg) at 2 hours. The results are depicted in Figure 3.
Anti-Nociceptive activity
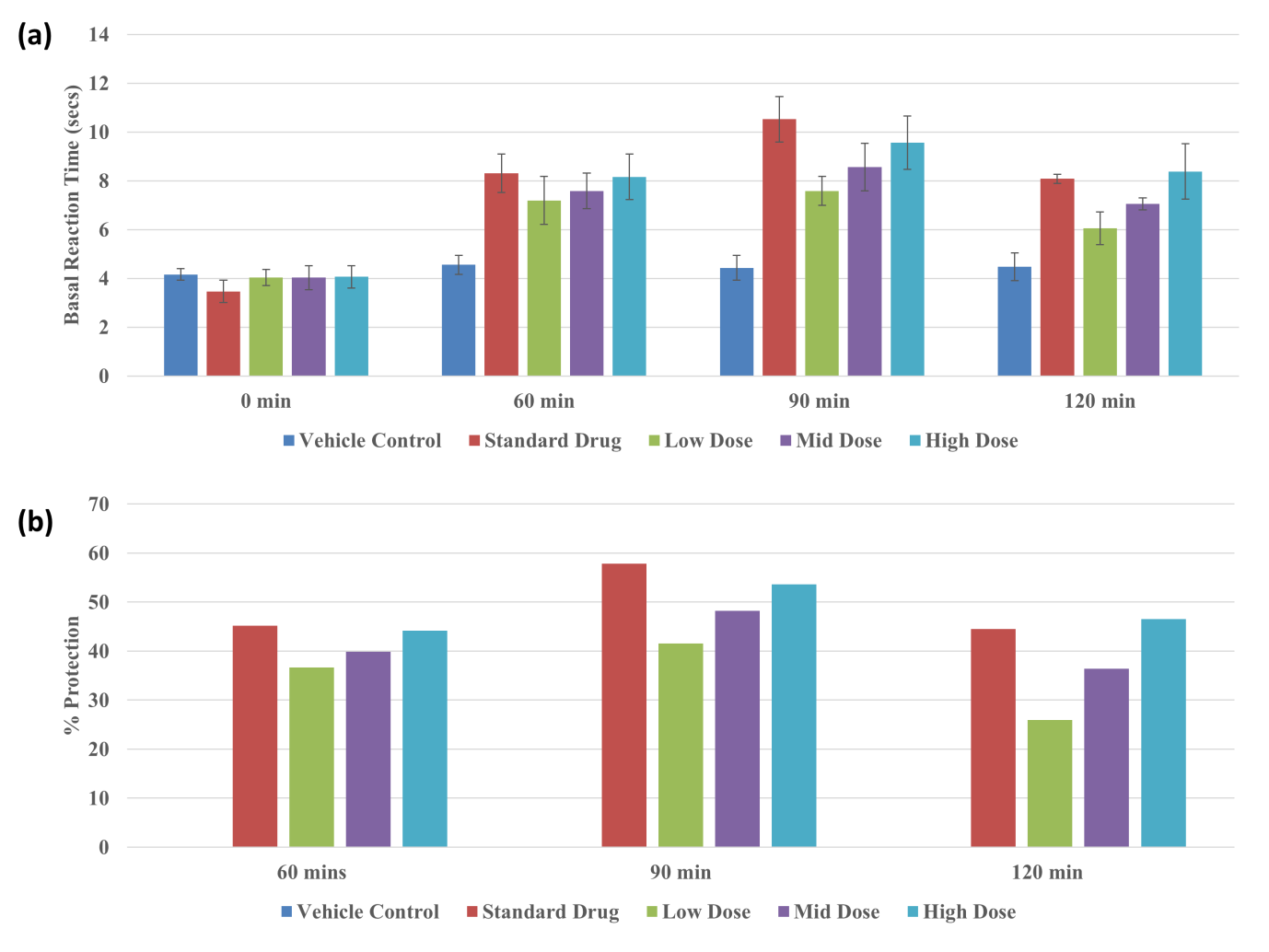
Central pain at the supraspinal and spinal levels was evaluated by the eddy’s hot plate thermal method. NUM was administered orally at 12, 24, and 48 mg/kg at low, moderate, and high doses, respectively. According to Eddy’s hot plate method, NUM increases the thermal stress tolerance of animals. At a low dose, the thermal stress tolerance was less significant at +90 minutes. In the mid-dose group, the difference was moderately significant at +60 minutes, whereas in the high-dose group, it was highly significant at +60 minutes and equal to that of the standard drug. This shows that after oral administration of the drug, the analgesic effect occurs after one hour, whereas for the standard drug administered iv, the analgesic effect occurs after 30 minutes. The results are depicted in Figure 4.
Anti-Spasmodic Activity
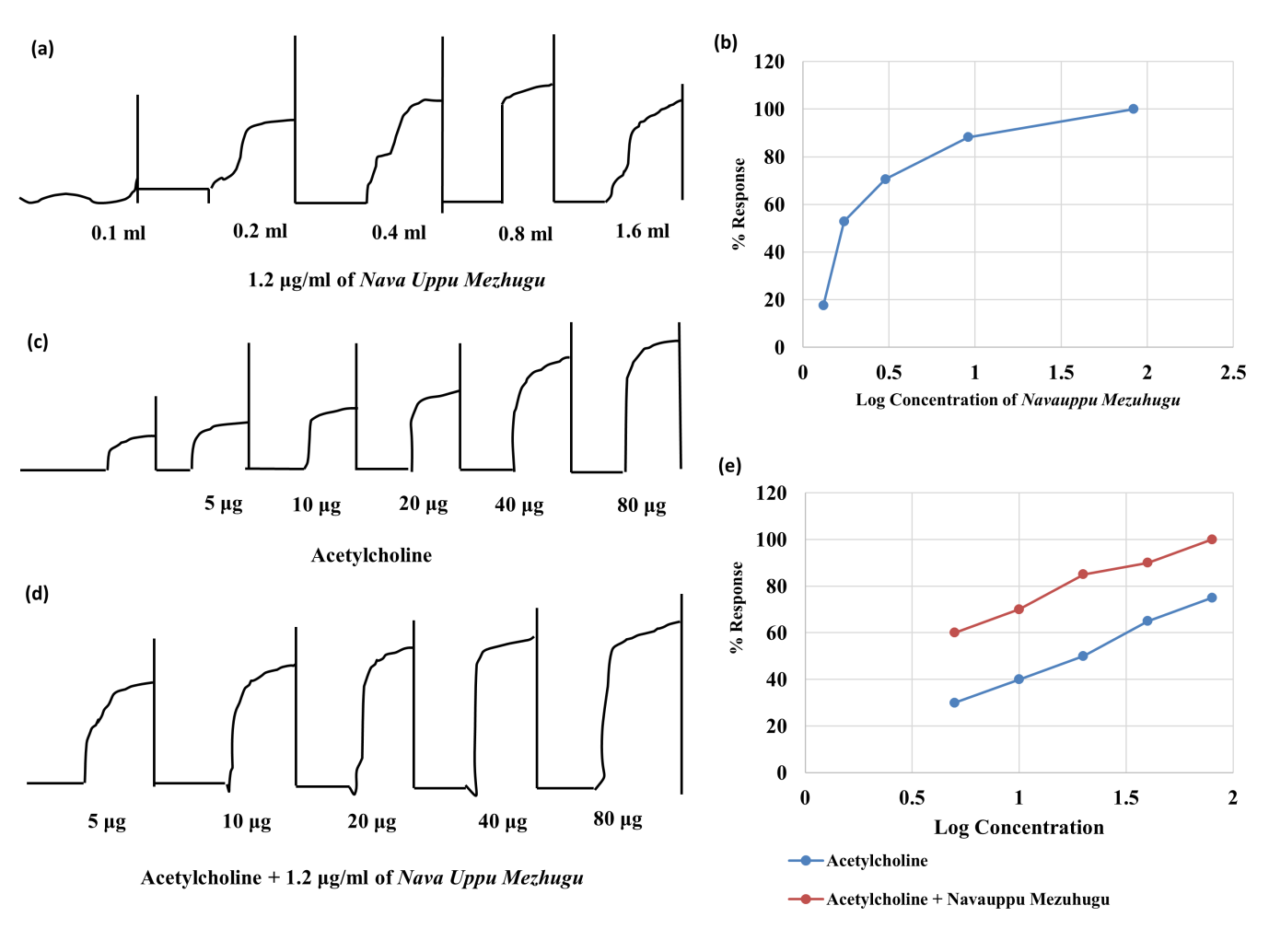
In terms of antispasmodic activity, when acetylcholine was administered to the ileum of rats, it produced a certain amount of normal contraction and a normal tissue response. This sample was used as a control. When 1.2 µg/ml NUM was administered at various concentrations, such as 0.2, 0.4, 0.6, 0.8, and 0.16 ml, contractions and tissue responses increased rather than decreased compared to those in the control group. This shows that the test drug NUM has no significant antispasmodic effect on the smooth muscles of the rat ileum.
Therefore, the test drug Nava Uppu mezhugu has significant antinociceptive (analgesic) activity and acute anti-inflammatory activity. The results are depicted in Figure 5.
The toxicity and safety profile of the test drug was determined previously by (Meenaloshini & Master of Thesis. National Institute of Siddha, Chennai - 47., 2019) as a master’s thesis but has not been published thus far (Meenaloshini & Master of Thesis. National Institute of Siddha, Chennai - 47., 2019).
CONCLUSION
The test drug Nava Uppu mezhugu is a promising polyherbo-mineral formulation that is practiced by traditional healers as well as Siddha practitioners and is regularly used for several ailments, especially under inflammatory conditions. In this study, standardization methods such as physicochemical analysis, chemical analysis, and modern instrumental analysis, such as FTIR and ICPOES, were evaluated. These results prove that their eminent structure, elemental composition, and physico-chemical characteristics have been documented. This reveals that the drug purity, safety, and shelf life were validated, thus proving its safety for consumption. According to reverse pharmacological studies, pharmacological activities such as anti-inflammatory and antinociceptive activities have shown significant results. Anti-spasmodic activity in the smooth muscles of animal models is not significant. Based on the results of these studies, Nava Uppu mezhugu is therapeutically effective and could be indicated for the treatment of RA and other pain- and inflammation-related diseases. Future clinical trials are needed to further substantiate the therapeutic efficacy and safety of NUM in diverse populations and elucidate the molecular mechanisms underlying its anti-inflammatory and antinociceptive effects to enhance understanding and optimize its use.
Ethical approval
Before initiating the preclinical evaluation, the study procedure was granted ethical approval by the Institutional Animal Ethics Committee (IAEC) of the National Institute of Siddha, Chennai. All procedures adhered to the guidelines and ethical principles for animal experimentation under appropriate care and control. The IAEC approval number for this study is NIS/IAEC-22/R02/16112021/16, dated 16.11.2021.
Author contributions
JT, SA, MD, and SSN: literature search, data collection, and analysis. JJT, SSN and TRV: conceptualization, study execution and supervision of the study. JT and SA: Manuscript preparation. JT, SA, MD, SRJ, SE, SSM and SSN: revision and proof reading of the article. All authors read and approved the final manuscript.