Introduction
Toxicity is a serious concern for anticancer medications. Organ toxicity exists in some of the most popular chemotherapeutic medications. One of the most often used anticancer medications is 5-fluorouracil (5-FU). It is used alone or in conjunction with other chemotherapeutic medications to treat a wide range of malignancies, including colon, breast, skin, and head and neck cancer (Argilés et al., 2020; Chen et al., 2019; Moro et al., 2019). Upon intracellular activation, the cytotoxic effect of 5-FU primarily occurs by impeding cellular thymidylate synthase, thereby impeding DNA replication and RNA synthesis by integrating its metabolites into RNA (Peters et al., 1994; Silverstein, Valdivia, Visa, & N, 2011). 5-FU is metabolized in the liver to dihydrouracil, which further decomposes into alpha-fluoro-beta alanine, urea, ammonia, and carbon dioxide, leading to hepatotoxicity and nephrotoxicity (El-Hoseany & M, 2012). Anticancer medications cause a multitude of adverse effects because of their toxic effects on normal cells/tissues, including nausea, vomiting, anorexia, diarrhoea, oral mucositis, and numbness. These adverse effects frequently reduce patients' quality of life (QOL) and may sometimes render it challenging to continue chemotherapy or chemoradiotherapy (Akin, Can, Aydiner, Ozdilli, & Durna, 2010). Even though numerous effective methods have been created to treat or prevent these adverse effects, they are still insufficient (Hershman et al., 2014; Jordan, Gralla, Jahn, & Molassiotis, 2014). It is therefore necessary to treat or avoid these adverse effects using an alternative or novel strategy. Herbal medicines are gaining popularity due to their pharmacological properties, few side effects in clinical trials, and low cost.
Thymoquinone (TQ; 2-isopropyl-5-methylbenzo-1,4-quinone), derived from the oil of Nigella sativa seeds, has exhibited preventative and therapeutic advantages across a diverse spectrum of diseases, including cancer (Gomathinayagam1, Ha1, & Jayaraman1, 2020), cardiovascular disease (Ali, Hashim, Shiekh, Majid, & Rehman, 2022), neurological disorder (Jakaria et al., 2018), respiratory disorder (Boskabady et al., 2021), gastrointestinal disorder (Aycan et al., 2018), and metabolic disorder (Razavi & Hosseinzadeh, 2014). TQ is a bioflavonoid and is therefore recognized to safeguard organs from oxidative damage brought on by a number of free radical-generating substances (Gholamnezhad, Havakhah, & Boskabady, 2016).
Hesperidin (HESP; 3,5,7-trihydroxy flavanone-7-rhamnoglucoside), a bioflavonoid present in citrus fruits (Abdelgawad et al., 2024), has been associated with various biological and pharmacological effects. It possesses anti-inflammatory (Tejada et al., 2017), antioxidant, and anticarcinogenic properties (Roohbakhsh, Parhiz, Soltani, Rezaee, & Iranshahi, 2015).
Although TQ and HESP have been shown to be effective against anticancer drug-induced toxicity, research on their impact on 5-FU induced nephrotoxicity is not yet reported. Therefore, the study sought to evaluate whether TQ and HESP could provide protection against 5-FU induced nephrotoxicity.
Materials and Methods
Chemicals
We procured 5-FU, TQ, and HESP from Sigma, St. Louis, MO (USA). Fetal Bovine Serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), Phosphate Buffer Solution (PBS), Dimethyl Sulfoxide (DMSO), Trypsin, and all necessary laboratory apparatus were acquired from Hi-media. All additional chemicals used met high analytical standards.
Cell line
Human Embryonic Kidney (HEK293) cell line were obtained from NCCS (National Centre for Cell Sciences, Pune, India).
Cell Viability
HEK293 cells, derived from human embryonic kidneys, were cultivated in DMEM containing 10% FBS, 1% antibiotic-antimycotic solution, and 1% L-Glutamine. When the cells reached approximately 70% confluence, they were sub-cultured using trypsinization and maintained in a controlled environment at 37°C with 5% CO2. For the MTT assay, HEK293 cells were seeded onto 96-well microtiter plates at a density of 5000 cells per well and then placed in an incubator set at 37°C with 5% CO2. After adherence, the cells were treated with varying concentrations of 5-FU, TQ, and HESP (5, 10, 25, 50, 100, and 200µg/mL) for a duration of 24 hours. Following treatment, MTT reagent at a concentration of 1 mg/mL was added to each well and incubated for an additional 4 hours. The resulting formazan crystals were dissolved in DMSO, and absorbance measurements were obtained at 570 nm using a multimode microplate reader (FluoSTAR Omega, BMG Labtech). Subsequently, calculations were performed to determine cell viability percentage and IC50 values (Shinde, Jain, Cheke, Surana, & Gunjegaonkar, 2021). The values of IC50 so obtained were used to calculate percentage cell viability of compounds in combination.
Apoptosis detection
Apoptosis was detected using Acridine Orange and Ethidium Bromide (AO-EB) dual staining. Cells were incubated with AO-EB solution at a ratio of 2 µg/ml each for 15 minutes at 37°C in the dark. After staining, excess dye was washed off with PBS, and the cells were observed for apoptosis using a fluorescence imager (ZOE, BioRad) under both green and red channels (Mohiyuddin, Naqvi, & Packirisamy, 2018).
Animal studies
Experimental animals
Male Wistar albino rats, weighing in the range of 170 to 200 grams were procured from the animal facility at Yenepoya University, Mangaluru. All experimental procedures followed the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines to enhance the quality and reproducibility of animal research. The study protocol was approved by the Institutional Animal Ethics Committee (Approval No: YU/IAEC/13/2022). The rats were accommodated in well-ventilated plastic cages under standardized conditions, maintaining a temperature of 22±2°C and a 12-hour light-dark cycle. They were fed a standard pellet diet and had continuous access to water. Before the initiation of the study, a two-week acclimatization period was provided to the rats. Before treatment administration, food was withheld from the animals for 12 hours. All animal handling procedures were conducted in strict accordance with the guidelines established by the Committee for Control and Supervision of Experiments on Animals (CCSEA), ensuring appropriate care and welfare.
Experimental design
The animals in the study were distributed randomly into seven groups, with each group consisting of six animals, as detailed below:
Group 1 (Vehicle)- administered saline for 8 days by oral gavage (Mohamed & Safwat, 2016).
Group 2 (5-FU)- received saline orally for 8 days, followed by a single intraperitoneal injection (I.P) of 5-FU (150 mg/kg body weight) on day 5 (Mohamed & Safwat, 2016).
Group 3 (TQ) - received TQ at a dose of 100 mg/kg orally for eight days (Adalı et al., 2016).
Group 4 (HESP) - received HESP at a dose of 100 mg/kg orally for eight days (Khedr, 2015).
Group 5 (TQ+5-FU) - received TQ (100mg/kg for eight days) and single dose of 5-FU on 5th day (150mg/kg, BW, I.P).
Group 6 (HESP+5-FU) - received HESP 100 mg/kg orally for 8 days and single dose of 5-FU on 5th day (150mg/kg, BW, I.P).
Group 7 (TQ+HESP+5-FU) - received HESP 50mg/kg + TQ 50mg/kg orally for 8 days and single dose of 5-FU on 5th day (150mg/kg BW, I.P).
Samples collection and storage
The animals were put under anesthesia for blood collection, 24 hours after the final treatment dose and subsequently euthanized using a high-dose combination of ketamine hydrochloride and xylazine (75+20mg/kg). Blood samples were drawn via cardiac puncture and serum was isolated by centrifuging them at 3,000 rpm, which was then stored at -80°C. Kidney samples were also frozen at -80°C for the evaluation of oxidative stress markers, antioxidants, and inflammation.
Assessment of Serum Kidney Function Markers
Serum creatinine and blood urea nitrogen (BUN) levels were measured using commercial kits (Accurex Biomedical, Mumbai, India) following the manufacturer's instructions.
Assessment of Kidney Tissue Oxidative Stress Markers
Malondialdehyde (MDA) and myeloperoxidase(MPO) as an oxidative stress marker, along with the antioxidant markers, reduced glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) in kidney tissue, were measured utilizing Quantikine ELISA kits (R&D Systems Inc., USA), following the manufacturer's instructions.
Statistical Analysis
All results were expressed as the Mean ± SD. Statistical comparisons between different groups were done using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer multiple comparison test, to judge the difference between various groups. Significance was considered at P<0.05. All graphs were plotted using Graph pad Prism 8 software (San Diego, CA. USA).
Results
In vitro effect of 5-Fluorouracil, Thymoquinone and Hesperidin on the viability of HEK-293 cells
To evaluate the cytotoxicity of 5-FU, TQ and HESP in cultured normal cells, HEK293 cells were exposed to various concentrations (5-200 µg/mL) of 5-FU, TQ and HESP alone and cell viability was determined using MTT assay for 24h to calculate dose-response curves (Figure 1) . 5-FU showed no cytotoxicity in HEK-293 cells from 5 to 50 μg/mL but exhibited cytotoxicity at 100 to 200 μg/mL. No significant cytotoxicity was observed in HEK-293 cells at 5 to 100μg/mL concentrations of TQ and HESP, but they exhibited cytotoxicity at 200μg/mL. The results indicated that cell viability significantly decreased with increasing concentration.
The IC50 value of 5- Fluorouracil, Thymoquinone and Hesperidin were calculated after individual treatment (Table 1 ). The value so obtained was used to calculate percentage cell viability of compounds in combination as determined by MTT assay. To determine percentage cell viability in combination, 5-FU was used at IC50 concentration. TQ and HESP were used at 1/4th of IC50 concentration, that is; 63.3μg/mL and 51.86μg/mL respectively. In HEK-293 cells treated with combination of compounds at IC50 concentration of 5-FU and 1/4th of IC50 concentration of TQ and HESP, the cell viability was in the following order: (TQ+HESP+5-FU) > (TQ+5-FU) > (HESP+5-FU) (Figure 2). The results suggested that combination of both TQ and HESP improved cell viability when compared to treating alone against 5-FU. The above same concentration was used for further experiment, that is, to detect apoptosis using Acridine orange: Ethidium bromide dual staining.
Figure 1
Dose response curve of 5-Fluorouracil (5-FU), Thymoquinone (TQ) and Hesperidin (HESP) against HEK-293 cells treated with different concentrations (5-200μg/mL) using MTT assay. The values are represented as Mean ± SD (n = 6). (a) p<0.001, (b) p<0.01, significant compared to the control group.

Apoptosis detection using AO-EB dual staining
AO-EB dual staining was performed on combination of compounds at the IC50 dose of 5- Fluorouracil and 1/4th of IC50 dose of Thymoquinone and Hesperidin. The results indicated that all the combinations showed viable cells as indicated by green fluorescence indicating absence of apoptosis (Figure 3).
Effect of TQ and HESP on Creatinine and BUN in treated rats
Figure 4 (A-B) illustrates the results of creatinine and BUN levels. The group induced with 5-FU showed a notable elevation in both creatinine and BUN levels in comparison to the control group. Nonetheless, simultaneous administration of TQ, HESP, and their combination with 5-FU led to a significant decrease in creatinine and BUN levels compared to the group treated with 5-FU alone. Additionally, among the groups treated with TQ+5-FU, HESP+5-FU, and their combination with 5-FU, the combination group demonstrated a significant improvement. Furthermore, when comparing between the TQ+5-FU and HESP+5-FU groups, the HESP+5-FU group displayed superior outcomes. These findings underscore the protective effects of TQ, HESP, and their combination against 5-FU induced toxicity.
Figure 4
The effect of TQ, HESP and 5-FU administration on level of renal enzymes andcomparison of different groups with each other (one-way Anova followed by Tukey-Kramer multiple comparision test). (A) Creatinine level (B) BUN level. All results are expressed as the Mean ± SD. (***) Significant difference from the control group at p < 0.001; (###) Significant difference from 5-FU group at p < 0.001; (@) Significant difference from TQ+5-FU at p<0.05; (@@@) Significant difference from TQ+5-FU at p<0.001; (&&&) Significant difference from TQ+5-FU at p<0.001; ($$$) Significant difference from HESP+5-FU at p <0.001. Abbreviations: BUN= Blood urea nitrogen; TQ= Thymoquinone; HESP= Hesperidin; 5-FU= 5-Fluorouracil.

Effect of TQ and HESP on tissue oxidative/nitrosative stress marker in treated rats
The results of tissue antioxidant/oxidative stress markers (SOD, GSH, CAT, MDA, MPO) are presented in Figure 6(A-E). The 5-FU-induced group showed a notable decrease in SOD, GSH, and CAT levels, alongside a significant increase in MPO and MDA levels compared to the control group. Treatment of TQ, HESP and their combination with 5-FU significantly restored renal SOD, GSH, CAT levels and significantly ameliorated MPO and MDA levels as compared to 5-FU group. Once again, the combination therapy showed the most significant improvement, with HESP demonstrating better efficacy compared to TQ when administered individually.
Figure 5
The effect of TQ, HESP and 5-FU administration on biochemical analysis and comparison of different groups with each other (one-way Anova followed by Tukey-Kramer multiple comparision test). (A) MDA level; (B) MPO level; (C) SOD level; (D) GSH level; (E) CAT level. All results are expressed as the Mean ± SD. (***) Significant difference from control group at p < 0.001; (###) Significant difference from 5-FU group at p<0.001; (@@) Significant difference from TQ+5-FU group at p<0.01; (@@@) Significant difference from TQ+5-FU group at p<0.001; (&&&) Significant difference from TQ+5-FU group at p<0.001; ($) Significant difference from HESP+5-FU group at p<0.05; ($$) Significant difference from HESP+5-FU group at p<0.01; ($$$) Significant difference from HESP+5-FU group at p<0.001.
Abbreviations: ns= non significant; SOD= Superoxide dismutase; MPO= Myeloperoxidase; MDA= Malondialdehyde; GSH= Reduced glutathione; CAT= Catalase; NO= Nitric oxide; TQ= Thymoquinone; HESP= Hesperidin; 5-FU= 5-Fluorouracil.


Effect of TQ and HESP on tissue inflammatory markers in treated rats
The results for tissue inflammatory markers (IL-6, IL-8, TNF-α) are illustrated in Figure 7(A-C). The group induced with 5-FU displayed a significant increase in all three inflammation markers compared to the control group. However, upon treating with TQ, HESP, and their combination with 5-FU notably decreased IL-6 levels, IL-8 levels, and TNF-α levels in comparison to the 5-FU group. Notably, the combination group exhibited significant improvement when compared to the TQ+5-FU and HESP+5-FU groups, with the HESP+5-FU group demonstrating superior outcomes over the TQ+5-FU group.
Figure 6
The effect of TQ, HESP and 5-FU administration on pro-inflammatory markers and comparison of different groups with each other (one-way Anova followed by Tukey-Kramer multiple comparision test). (A) IL-6 level (B) IL-8 level (C) TNF-α level. All results are expressed as the Mean ± SD. (***) Significant difference from control group at p < 0.001; (###) Significant difference from 5-FU group at p<0.001; (@@@) Significant difference from TQ+5-FU group at p<0.001; (&&&) Significant difference from TQ+5-FU group at p<0.001; ($) Significant difference from HESP+5-FU group at p<0.05. Abbreviations: ns= Non significant; IL-6= Interleukin 6; IL-8= Interleukin 8; TNF-α= Tumor necrosis factor; TQ= Thymoquinone; HESP= Hesperidin; 5-FU= 5-Fluorouracil.

Histopathological examination
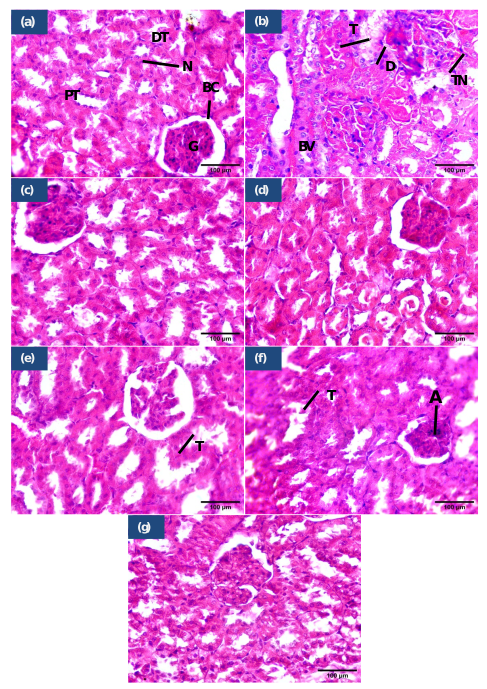
The kidney tissues from the control group demonstrated histological patterns consistent with normal morphology, while those from the 5-FU treated group exhibited severe pathological changes, including tubular degeneration, necrosis, and dilated blood vessels. Treatment with TQ +5-FU reduced tubular damage, while HESP+5-FU preserved renal structure better. Remarkably, TQ + HESP + 5-FU showed nearly normal tissues, indicating significant protection against 5-FU-induced renal toxicity (Figure 8).
Figure 7
Histopathological findings of kidney tissues. (a) control group showing normal renal histology, (b) 5-FU treated group showing disrupted of bowman’s capsule, tubular dilation, necrosis, dilated blood vessels packed with RBC, (c) TQ treated group showed normal bladder histology, (d) HESP treated group showed normal histology, (e) TQ+5-FU treated group showed mild injured renal tissues, (f) TQ+HESP treated group showed semi normal renal tissues, (g) TQ+HESP+5-FU treated group showed almost normal renal tissues. Abbreviations used: BC= Bowman's capsule; G= glomerulus; PT= proximal tubule; DT= distal tubule; N= nuclei; BV= blood vessels; T=tubular dilation; TN= tubular necrosis; A=glomerular atropy; D=disrupted Bowman’s capsule; TQ= Thymoquinone; HESP= Hesperidin; 5-FU= 5-Fluorouracil. (H&E; X400, scale bar= 100μm)
Discussion
5-FU is a chemotherapy medication widely employed in the treatment of various cancer types owing to its efficacy against a spectrum of human malignancies (Prince, Cameron, Fathi, & Alkousakis, 2018). However, despite its therapeutic benefits, it is associated with significant nephrotoxic side effects (Badawoud, Elshal, Zaki, & Amin, 2017).
The molecular mechanism of 5-FU-induced nephrotoxicity primarily involves oxidative stress, inflammation, and apoptosis. 5-FU promotes excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), leading to lipid peroxidation, nitric oxide imbalance, and depletion of antioxidant enzymes like GSH, CAT, and SOD (Ranasinghe, Mathai, & Zulli, 2023). This oxidative stress triggers an inflammatory response by activating nuclear factor-kappa B (NF-Κb), which upregulates pro-inflammatory cytokines such as TNF-α and IL-6, further exacerbating renal damage (Rapa et al., 2021).
Additionally, oxidative stress disrupts mitochondrial function, leading to apoptosis. ROS-induced conformational changes in BCL2-associated X protein (Bax) cause its translocation to mitochondria, resulting in cytochrome c release and activation of Caspase-9 and Caspase-3, which drive cell death (Małyszko, Kozłowska, Kozłowski, & Małyszko, 2017). These molecular events collectively contribute to renal tubular injury, inflammation, and chronic kidney disease (CKD) progression (Q, Tong, Ren, He, & Cong, 2020).
In this study, we examined the protective effects of TQ, HESP, and their combination against 5-FU-induced nephrotoxicity through comprehensive in vitro and in vivo assessments. The rationale for using a combination of TQ and HESP alongside 5-FU in this study lies in their potential to mitigate chemotherapy-induced toxicity, and the exploration of novel therapeutic combinations.
Firstly, the cytotoxic effects of 5-FU, TQ, and HESP were evaluated in cultured normal HEK293 cells. The MTT assay revealed differential cytotoxicity profiles for the three compounds. 5-FU exhibited dose-dependent cytotoxicity, with no significant effects observed at lower concentrations (5-50μg/mL) but pronounced cytotoxicity at higher concentrations (100-200μg/mL). Conversely, TQ and HESP demonstrated no significant cytotoxicity at concentrations up to 100μg/mL, but toxicity was evident at 200μg/mL. These findings indicate that while 5-FU may be less toxic at lower doses, caution must be exercised at higher concentrations. TQ and HESP, on the other hand, appear to have a wider safety margin in HEK293 cells at lower concentrations.
Further analysis involved calculating the IC50 values for each compound and assessing their combined effects on cell viability. Interestingly, when HEK-293 cells were treated with a combination of 5-FU at IC50 concentration, TQ and HESP at 1/4th of their respective IC50 concentrations, the combination of TQ and HESP significantly improved cell viability compared to treating with 5-FU alone. To validate these findings, apoptosis was assessed using AO-EB dual staining. Encouragingly, all combinations tested showed viable cells, indicated by green fluorescence and the absence of apoptotic features. This implies that the combination treatments did not induce significant apoptotic cell death in HEK293 cells under the conditions tested. Prior studies have shown that isolated compounds protect HEK293 cells from cisplatin-induced kidney damage, markedly enhancing cell viability in a dose and time dependent manner, while leaving normal cells unaffected (Dan et al., 2019; Jie et al., 2018; Q et al., 2020).
The current study examined the potential protective effects of TQ and HESP, alone and in combination, against toxicity induced by 5-FU in rats. Our findings revealed that 5-FU when given at toxic dose caused marked increase in creatinine and BUN levels, indicative of renal dysfunction, compared to the control group. These findings align with previous studies that also reported increased serum levels of creatinine and BUN in rats treated with 5-FU (Al-Asmari et al., 2016; Xiong et al., 2016; Yousef & Aboelwafa, 2017). Treatment with TQ, HESP, or their combination significantly attenuated these elevations, suggesting a protective effect against 5-FU-induced renal injury. Notably, the combination therapy exhibited superior efficacy, emphasizing a potential synergistic interaction between TQ and HESP in ameliorating chemotherapy-associated nephrotoxicity. A study conducted by Ali YA et al. (2023), found that the combined administration of rutin and hesperidin exhibited greater efficacy in mitigating chemotherapeutic drug-induced nephrotoxicity compared to when each compound was administered individually (Ali et al., 2023).
Several studies have indicated that TQ and HESP mitigate oxidative stress by decreasing the production of ROS and MDA, while simultaneously enhancing antioxidant levels such as SOD, GSH, and GPx in various models of chemotherapeutic drug-induced toxicity (Farooq et al., 2021; Gelen et al., 2021; Jia et al., 2022). Our study elucidated the impact of TQ and HESP on oxidative stress markers, crucial contributors to chemotherapy-induced tissue damage. We observed that 5-FU exposure resulted in a significant imbalance in antioxidant enzyme activity, characterized by decreased levels of SOD, GSH, and CAT, along with increased levels of MDA and MPO. However, treatment with TQ, HESP, or their combination successfully restored the antioxidant defense system and reduced oxidative stress, highlighting their potential to protect renal function during chemotherapy. Once again, the combination therapy displayed the most pronounced protective effects, underscoring its therapeutic superiority. In drug-induced nephrotoxicity, inflammatory mechanisms play a significant role, as oxidative stress and inflammation are closely intertwined. Pro-inflammatory mediators can instigate oxidative stress, while free radicals are regarded as mediators that initiate and perpetuate inflammation (Dobrek, 2023). Moreover, cytokines are pivotal in the molecular pathogenesis of drug-induced nephrotoxicity (Antar et al., 2022). 5-FU can induce an increase in pro-inflammatory cytokine levels, such as tumor TNF-α, IL-6, and IL-8 through several mechanisms. One mechanism involves the direct cytotoxic effects of 5-FU on cells, leading to cellular stress and damage (Abdellateif et al., 2020). Our investigation into inflammatory markers revealed a significant elevation in IL-6, IL-8, and TNF-α levels following 5-FU administration. However, treatment with TQ, HESP, or their combination attenuated these inflammatory responses, suggesting their anti-inflammatory properties. The observed reduction in pro-inflammatory cytokine levels highlights the potential of TQ and HESP in modulating immune-mediated renal injury associated with chemotherapy. Previous study reported the inhibitory effect of phytochemical compounds against pro-inflammatory cytokines in 5-FU induced nephrotoxicity (Gelen, Şengül, Yıldırım, & Atila, 2018). In another study conducted by Ansari MA et al. (2023), Sinapic acid protected against 5-FU nephrotoxicity by activating nuclear factor erythroid 2-related factor 2 and heme oxygenase 1 (Nrf2/HO-1) to boost antioxidants and reduce oxidative stress. It also inhibited NF-κB, lowering pro-inflammatory cytokines (TNF-α, IL-6) and oxidative damage while also suppressing apoptosis by preventing Bax mediated mitochondrial dysfunction and caspase activation (Ansari et al., 2023).Previously reported study described the molecular mechanism of TQ in reducing methotrexate (MTX) induced toxicity. MTX induced inflammation and apoptosis in the liver and kidneys by increasing TNF-α, NF-κB activation, COX-2 expression, and caspase-3, leading to cellular damage. When co-administered with TQ, these toxic effects were reversed, with TQ restoring organ function, improving histology, and reducing oxidative stress, inflammation, and apoptosis by downregulating key inflammatory markers and enzymes (El-Sheikh, Morsy, Abdalla, Hamouda, & Alhaider, 2015). Previous research has demonstrated that HESP mitigates paclitaxel (PTX)-induced liver and kidney toxicity by alleviating oxidative stress, inflammation, and apoptosis. It enhances antioxidant defenses, regulates inflammatory cytokines (such as TNF-α, IL-6), and suppresses apoptotic markers like Caspase-3 and Bax while promoting cell survival via Bcl-2. Furthermore, HESP activates protective pathways (Nrf2/HO-1) and decreases matrix metalloproteinases (MMP-2, MMP-9), highlighting its potential role in preventing PTX-induced organ damage (Ali et al., 2023).
The histological analysis revealed that kidneys treated with 5-FU exhibited severe pathological changes, including tubular degeneration and necrosis, whereas those in the control group showed normal histology. Treatment with TQ + 5-FU reduced tubular damage, while HESP + 5-FU preserved renal structure more effectively. Notably, the combined therapy of TQ + HESP + 5-FU demonstrated nearly normal tissue architecture, indicating significant protection against 5-FU-induced renal toxicity. similar changes in 5-FU treated group was reported in earlier studies (Gelen et al., 2021; Gelen et al., 2018).
The potential clinical application of TQ and HESP as adjunctive treatments in chemotherapy shows promise for reducing nephrotoxicity and improving patient outcomes. Their antioxidant and anti-inflammatory properties could help mitigate renal damage while preserving chemotherapy efficacy. However, translating these findings into human medicine presents challenges, such as the lack of clinical trials on safety, dosage, and pharmacokinetics in cancer patients. Potential interactions with chemotherapy drugs also need thorough investigation. Furthermore, bioavailability and metabolism in humans may differ from animal models. Additional research is needed to clarify the molecular mechanisms behind these effects and assess their potential clinical translation. This study focuses on the immediate effects of these compounds, leaving long-term safety and efficacy unexplored, underscoring the need for further research on prolonged use and delayed effects.Top of Form
Conclusion
In summary, our study demonstrates that TQ and HESP, alone or in combination, effectively mitigate 5-FU-induced nephrotoxicity by reducing oxidative stress, inflammation, and preserving renal tissue architecture. These findings suggest the therapeutic potential of TQ and HESP as adjunctive treatments to alleviate chemotherapy-induced kidney damage, warranting further investigation for clinical translation.




