Background
Diabetes mellitus is a heterogeneous metabolic disorder that implicates the body's inability to manage glucose and insulin homeostasis, disrupting carbohydrate, protein, and fat metabolism (Cho et al., 2018). Uncontrolled Diabetes mellitus may lead to microvascular or macrovascular complications like neuropathy, nephropathy, retinopathy, cardiovascular diseases, stroke and peripheral vascular diseases. Bruising, gangrene or non-healing injuries that ultimately end with amputation as a part of peripheral vascular diseases that may worsen the QOL of the diabetic population (Deshpande, Harris-Hayes, & Schootman, 2008). World Health Organisation (WHO) stated that Diabetes mellitus caused nearly 1.5 million deaths in 2019, and 48% of it occurred before 70 years. Hyperglycaemia accounted for 4,60,000 Medico Renal Disease (MRD) deaths and 20% of cardiovascular deaths that occur globally. The age-standardized mortality rate due to Diabetes increased by 3% in high-income countries and 13% in lower and middle-income countries between 2000 and 2019 (WHO, 2024). WHO estimates that around 422 million people suffer from Diabetes mellitus, and India and China are the major contributors to the global diabetic burden (Saeedi et al., 2019). The National Family Health Survey 5 (NFHS 5) reported a higher prevalence (15.6 vs 13.5 %) of Hyperglycaemia among men than women, and 15% of the diabetic population in India remain undiagnosed or unaware of their health status. The out-of-pocket expenses for Diabetes and its related complications were around 114.4 USD for individuals and 10.1 billion for Indian diabetes expenditure in 2021 (Sahadevan et al., 2023).
In 2019, the International Diabetes Federation reported that the number of people with Diabetes was estimated to be 463 million and would increase to 700 million by 2045 (Saeedi et al., 2019). Physical inactivity, concomitant sedentary behaviour modification and unhealthy dietary habits exacerbate T2DM and obesity (Kusirisin, Jaikang, Chaiyasut, & Narongchai, 2009). Type-2 diabetes mellitus is triggered by Genetic and environmental factors, and a pro-inflammatory state in peripheral insulin resistance contributes to the functional failure of ß cells (Kahn, Cooper, & Prato, 2014; Murea, Ma, & Freedman, 2012). Insulin resistance conditions further cause increased reactive oxygen species (ROS) molecules due to excessive accumulation of glucose and circulatory free fatty acids in adipose tissue (Rains & Jain, 2011).
Dyslipidemia and Diabetes mellitus are closely interrelated and frequently coexist among Asian populations. Factors like a high saturated fat diet, overweight/obesity, and familial history of high cholesterol eventually lead to insulin resistance and increase the risk of Type 2 Diabetes mellitus (Care, 2019). Metabolic syndrome is a cluster that includes metabolic dysregulations like insulin resistance, Dyslipidemia, central obesity and Hypertension (Swarup, Ahmed, Grigorova, & Zeltser, 2024). Chronic Hyperglycemia leads to oxidative stress-induced increased ROS production that disrupts glucose regulation by impaired beta cell function and insulin resistance. Therefore, a drug that effectively targets these issues while also managing oxidative stress and associated complications is a priority and a necessity. Conventionally, many oral hypoglycemic and exogenous insulin supplementations are used for managing type 2 diabetes along with sedentary modification (Pepato et al., 2005). According to World Health Organization reports, many people globally still rely upon conventional medicines for their well-being needs (Dias, Urban, & Roessner, 2012). The Siddha system of medicine has been well-established and practised enormously in southern India and in recent times worldwide (Muthiah, Ganesan, Ponnaiah, & Parameswaran, 2019). Diabetes mellitus and its complications can be correlated with signs and symptoms of Iḷippu nīr or Matumēkam and Avattaikaḷ. Siddha medicines illustrated various herbal, mineral, herbo-mineral and metallic formulations for T2DM (Matumēkam) and its complications (Avattaikaḷ) (Muthaliyar & K, 2012). Among the various Siddha formulations, Cuṇṭai Vaḷḷal Cūraṇam (CVC) (Muthaliyar & K, 2009) is recognized for its multifaceted health benefits, offering potential solutions to modern-day metabolic disorders. This article sets the stage for a comprehensive exploration of the pharmacological potential of CVC, integrating traditional Siddha wisdom with contemporary scientific analysis. Consequently, the main objective of this research is to assess the antidiabetic, Antihyperlipidemic, and antioxidant properties of the traditional Siddha formulation Cuṇṭai Vaḷḷal Cūraṇam through targeted inhibition assays.
Methods
Study drug
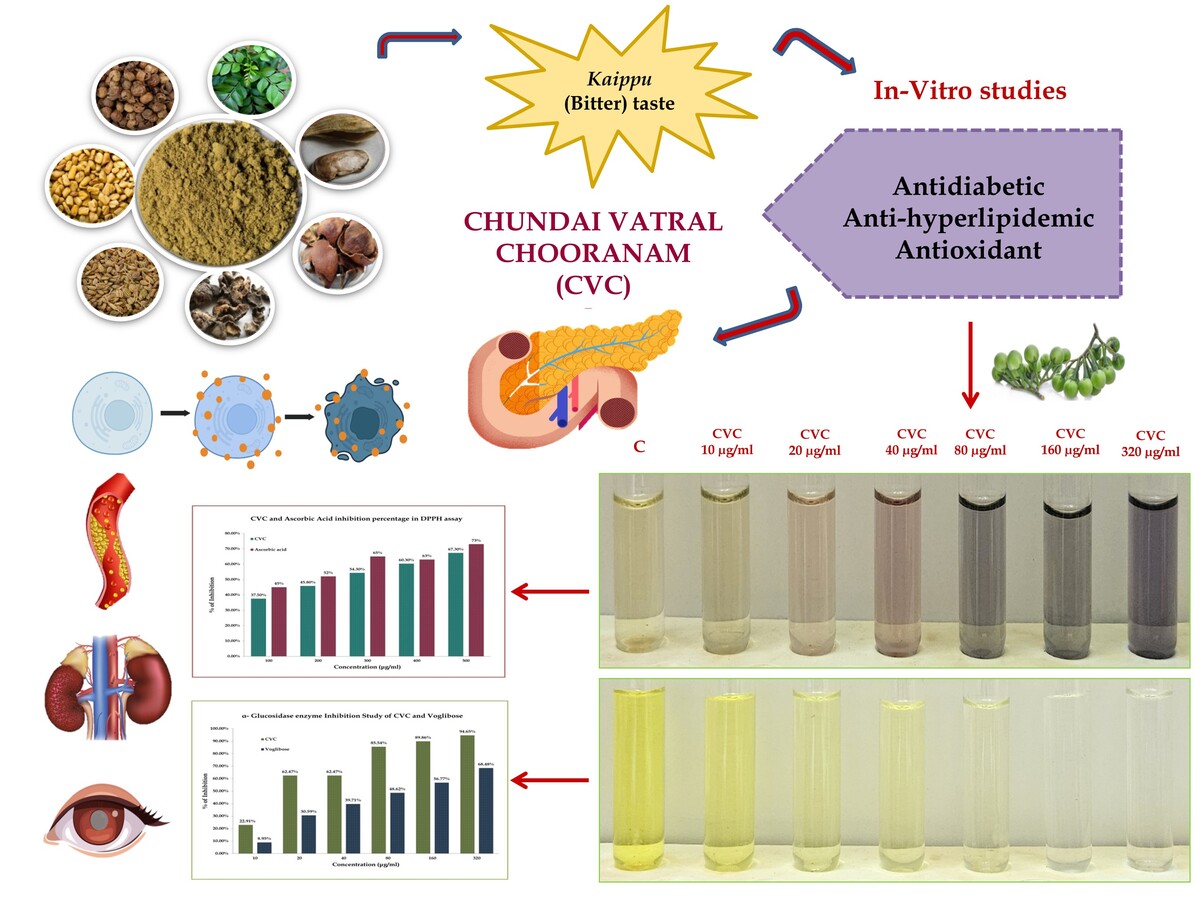
The formulation was procured from IMPCOPS (The Indian Medical Practitioners Co-Operative Pharmacy and Stores Ltd.) and authenticated by the Research Officials. The ingredients of the drug CVC are tabulated in Table 1 and Figure 1. The powder was mixed with distilled water and used for the following experiment. The spectrophotometric approach was utilized to prepare and assess the dissolved sample.
Table 1
Composition of Cuṇṭai Vaḷḷal Cūraṇam (CVC).
Determination of Antidiabetic Activity
α-Amylase Inhibition Study
The α-amylase inhibitory activity of the test sample (CVC) was evaluated using a slightly modified standard procedure (Unuofin, Otunola, & Afolayan, 2018). Different concentrations of the test sample (10, 20, 40, 80, 160, and 320 µg/mL), the reference standard (Acarbose), and a control (without the test sample or standard) were mixed with a solution of α-amylase (100 µL, 0.1 mg/mL). The mixtures were pre-incubated at 37°C for 15 minutes. Subsequently, 100 µL of starch solution was added to initiate the enzymatic reaction, and the incubation was continued at 37°C for 60 minutes. Each tube was filled with 100 µL of iodine reagent and 10 µL of 1 M HCl to stop the reaction. At 565 nm, the final solution's absorbance was measured.
The α-amylase inhibitory activity was calculated using the formula:
In this method, the OD of control refers to the absorbance measured for the control sample (lacking the test sample or standard). At the same time, the OD of the Test denotes the absorbance of the sample at different concentrations. The α-amylase inhibitory potential of CVC is determined by comparing the absorbance values of the test samples with those of the control (Unuofin et al., 2018).
α-Glucosidase Enzyme Inhibition Study
The effect of the test sample (CVC) on α-glucosidase activity was evaluated following the method described by Apostolidis et al. p-nitrophenyl glucopyranoside (pNPG), the substrate was made at pH of 6.8 in a 100 mM phosphate buffer. Various concentrations of the test extracts (10, 20, 40, 80, 160, and 320 µg/mL) were pre-incubated for 10 minutes in 200 µL of α-glucosidase solution. The pre-incubated solutions were mixed with 400 µL of a 5.0 mM pNPG solution to start the reaction. After 20 minutes of incubation at 37°C, 1 mL of 0.1 M Na2CO3 was added to the reaction mixtures to stop the activity. A UV-VIS spectrophotometer was used to measure the absorbance at 405 nm to quantify the release of 4-nitrophenol, which results in a yellow tint. Voglibose was used as a positive control. The α-glucosidase inhibitory activity was calculated using the formula:
In this method, Abs sample denotes the absorbance of the reaction mixture containing the test sample, while Abs Control denotes the absorbance of the control reaction (without the test sample). By determining the decrease in absorbance, which is equivalent to the yellow 4-nitrophenol generated from the pNPG substrate, the inhibition of α-glucosidase activity can be determined (Apostolidis, Kwon, & Shetty, 2007).
Determination of Anti-Hyperlipidemic activity
β-Hydroxy β-methylglutaryl-CoA (HMG-CoA inhibitory assay)
The spectrophotometric approach evaluated the test sample's (CVC) HMG-CoA reductase inhibitory assay activity. A reaction mixture including 60 µl of NADPH (400 µM), HMG-CoA Substrate (400 µM), and potassium phosphate buffer (100 mM, pH7.4) containing KCl (120 mM), EDTA (1 mM), and DTT (5 mM) was mixed with extract samples of various concentrations. After 10 minutes of incubation at 37°C, the absorbance at 340 nm was measured for the reaction mixture. Atorvastatin was used as a positive control.
This formula compares the optical density of the test sample to that of the control to quantify the test sample's inhibitory effect on HMG-CoA reductase (Baskaran et al., 2015).
1: 2,2-Diphenyl -1 - picryl hydrazyl assay (DPPH)
In the DPPH assay, the DPPH free radical transforms into a stable, non-radical form by reacting with electrons or hydrogen atoms. The sample extract is prepared as a stock solution at 100 μg/mL (10 mg/100 mL). The final concentrations of 10, 20, 40, 60, 80, and 100 μg/mL are serially diluted to 1 mL, 2 mL, 4 mL, 6 mL, 8 mL, and 10 mL solvents.
The same method is used to make ascorbic acid a standard. Each test tube combines one millilitre of a 0.3 mM DPPH solution with 2.5 milliliters of the sample or standard. Using methanol as the blank, absorbance is measured at 517 nm using a UV spectrophotometer following a 15-minute incubation period at 37°C. This process calculates the antioxidant activity of the sample using the following formula:
This calculation provides the percentage of DPPH radicals scavenging activity by the sample or standard, indicating its antioxidant potential (Sivakkumar, Juliet, & Meenakumari, 2024).
2: 2,2′-Azino-Bis 3-ethylbenzothiazoline-6-sulfonic acid assay (ABTS)
For the ABTS Assay, the ABTS radical solution was made by mixing 05 mL of a 07 mM ABTS solution with 88 μL of a 140 mM potassium persulfate solution. The resulting mixture was mixed with water at a ratio of 1:44. 100 μL of the CVC solution was combined with 100 μL of the ABTS reagent for each CVC concentration (10–100 μg/mL), and the mixture was allowed to react. For comparison, Ascorbic acid was used as a positive control (Sivakkumar et al., 2024).
This formula measures the ability of the test sample to scavenge ABTS free radicals, which is indicated by a reduction in absorbance.
Results
Antidiabetic activity
It was observed from the results that CVC significantly inhibited the alpha-amylase enzyme, with a maximum inhibition of 63.08% and an IC50 value of 24.01μg/mL. In contrast, the standard drug achieved a 97.37% inhibition with an IC50 value of 3.21μg/mL.
CVC has a maximum inhibition of 68.48% against alpha-glucosidase enzyme with an IC50 value of 92.52μg/mL, and the standard drug Voglibose has 94.65% inhibition with an IC50 value of 17.63μg/mL is depicted in Figure 2, Figure 3.
Antihyperlipidemic activity
CVC demonstrates a notable level of inhibition, reaching 63.54% at 120 μg/mL concentration, with an IC50 value of 68.58μg/mL, and the standard drug Atorvastatin showed maximum inhibition of 94.78 at 120 μg/mL with an IC50 value of 17.46μg/mL is depicted in Figure 4.
Antioxidant activity
The extract of CVC showed the highest DPPH scavenging activity (67.3%) at 100µg/ml with an IC50 value of 51.79 µg/ml, and the Ascorbic acid (Standard) showed the highest percentage of inhibition (72%) at 100µg/ml with IC50 value of 16.63 µg/ml.
Based on the ABTS assay results, CVC exhibits a maximum inhibition of 74.7% at 500µg/mL, with an IC50 value of 118.4 µg/mL. In comparison, the standard shows a maximum inhibition of 72% at 500µg/mL and has an IC50 value of 218.4 µg/mL. This indicates that CVC is more effective in inhibition and has a lower IC50 value, signifying it is more potent than the standard depicted in Figure 5, Figure 6.
Discussion
Type II diabetes is the more prevalent form of Diabetes that is often undiagnosed or untreated, and more than 90% have been associated with Dyslipidemia, which may accelerate the development of atherosclerotic cardiovascular disease, end-stage renal disease and hypertension (An et al., 2023; Nowakowska et al., 2019). In T2DM, hyperglycemia-induced glycation in extracellular proteins like elastin, laminin, and collagen leads to the formation of Advanced glycolic end-products (AGEs) that accumulate in tissues of the eye lens, vascular walls, and basement membranes. These modifications are associated with various diabetic complications, including Cataracts, Microangiopathy, Atherosclerosis, Nephropathy, etc. Modern oral antidiabetic drugs include biguanides, sulfonylureas, meglitinides, TZDs, DPP-4 inhibitors, SGLT2 inhibitors and α-glucosidase inhibitors (Chaudhury et al., 2017). Long-term use of these medications may lead to complications such as injection site reactions, gastrointestinal issues, cobalamin deficiency, dizziness, liver enzyme elevation, edema, respiratory infections, and headaches. Focusing on the multifactorial range of complications necessitates a cost-effective, comprehensive therapeutic management with minimal side effects. This in-vitro study on the Siddha polyherbal formulation, Cuṇṭai Vaḷḷal Cūraṇam (CVC), reveals its promising potential in managing T2DM through its notable antidiabetic, antihyperlipidemic, and antioxidant properties.
Calcium-containing α-amylase in the oral cavity, Serum α-amylase in the gut, and α-glucosidase in the small intestine maintain postprandial Hyperglycemia by inhibiting carbohydrate digestion and glucose regulation (Sahu, Upadhyay, & Panna, 2014). CVC exhibited potent inhibitory effects on both α-amylase and α-glucosidase enzymes regarding antidiabetic activity. Specifically, CVC achieved a maximum α-amylase inhibition of 93.08% at a concentration of 320μg/ml, with an IC50 value of 23.01μg/ml. This substantial inhibition indicates that CVC effectively impedes the enzymatic breakdown of complex carbohydrates into polysaccharides, oligosaccharides, disaccharides and monosaccharides. By slowing down this process, CVC can reduce the digestion and absorption rate of carbohydrates, thereby mitigating postprandial glucose spikes - a key factor in managing blood sugar levels.
Additionally, CVC demonstrated a maximum α-glucosidase inhibition of 94.65% at 320μg/ml, with an IC50 of 92.52μg/ml. α-Glucosidase breaks down disaccharides into glucose, which is absorbed into the bloodstream (Rajalakshmi, Christian, Priya, & Gladys, 2015; Truscheit et al., 1981). By inhibiting this enzyme, CVC can decrease the conversion of disaccharides into glucose, resulting in a slower and more controlled release of glucose into the bloodstream. This mechanism further supports its role in blood glucose management, as it helps to maintain postprandial Hyperglycemia (Baron, 1998; Laar, 2008).
Diabetic Dyslipidemia is defined as high plasma triglyceride concentration with low HDL cholesterol levels and increased concentration of small dense LDL cholesterol particles. Diabetes mellitus associated with lipid changes is attributed to insulin resistance-induced free fatty acid flux (Mooradian, 2009). Regarding Antihyperlipidemic activity, CVC demonstrated significant inhibition of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase, achieving a maximum inhibition of 63.54% at a concentration of 120μg/ml. HMG-CoA reductase plays an essential role in cholesterol biosynthesis by catalysing the rate-limiting step in the production of cholesterol within the liver. Specifically, this enzyme converts HMG-CoA to mevalonate, a precursor of cholesterol (Friesen & Rodwell, 2004). This step is crucial because it is one of the primary regulatory points in the cholesterol synthesis pathway, determining the rate at which cholesterol is produced. When CVC inhibits HMG-CoA reductase, it blocks this critical step, disrupting the cholesterol synthesis process. This inhibition leads to a decrease in the production of mevalonate and subsequent cholesterol. As a result, the liver's ability to produce cholesterol is diminished. This reduction in hepatic cholesterol synthesis subsequently lowers the blood's circulating cholesterol levels (Feingold, 2024; Trapani, Segatto, & Pallottini, 2012). By decreasing cholesterol production within the liver, CVC can help to manage high cholesterol levels, a key factor in hyperlipidemia and related cardiovascular conditions. This mechanism mirrors the action of statins, such as Atorvastatin, widely used to control cholesterol levels. Although CVC's inhibitory effect is less pronounced compared to the well-established statin drug Atorvastatin, which achieves a maximum inhibition of 94.78%, the substantial activity observed in CVC suggests it has the potential to contribute effectively to cholesterol management.
Recent research highlights the significant role of free radicals in the pathogenesis of Diabetes and, notably, in its complications (Caturano et al., 2023). Antioxidants, which neutralize free radicals, effectively mitigate the severity of diabetic complications. So, managing free radical damage through antioxidants and other strategies is crucial for reducing the impact of these complications and improving patient outcomes (Matough, Budin, Hamid, Alwahaibi, & Mohamed, 2012). CVC demonstrated considerable antioxidant activity in the DPPH and ABTS assays, achieving maximum inhibitions of 67.3% and 74.7%, respectively. The DPPH assay, which measures the ability to scavenge the DPPH radical, revealed that CVC's antioxidant potency was slightly lower than that of the standard ascorbic acid, which had a maximum inhibition of 73%. Despite this, CVC's antioxidant capacity is still significant, highlighting its effectiveness in neutralizing free radicals and potentially mitigating oxidative damage.
In contrast, CVC excelled in the ABTS assay, which achieved a maximum inhibition of 74.7%, outperforming the standard, which showed a maximum inhibition of 72%. CVC's superior performance in this assay underscores its robust ability to neutralize a range of reactive oxygen species. This dual efficacy in combating oxidative stress suggests that CVC could be particularly valuable in managing conditions associated with excessive oxidative damage, such as Diabetes and its complications. By effectively reducing oxidative stress, CVC may help to prevent or alleviate damage to cellular components, thus supporting overall health and potentially enhancing the management of chronic diseases.
As per the Siddha system of medicine, the treatment plan for Matumēkam relies on the basic principles like Mukkuḷḷam (three vital forces like Vali, Azhal and Iyam), Cuvai (Taste of the drug), Vīriyam (Potency of the drug), Vipākam (Post-digestive biotransformation of the drug) and Ceykai (Pharmacological activity of the drug). In Matumēkam to normalize the alleviated Aḳal and Aiyam and diminished Vai, CVC is an appropriate choice of drug due to its Kaippu (bitter), Tuvarppu (astringent), and Kārppu (pungent) taste (Shanmugavelu, 2014). As Siddha's text mentions, taste's characteristic features and properties are bitter - purifies and dries out the body secretion, astringent - blood purifier, pungent - appetizer and cardiac tonic (Venugopal, 2014). Bitter taste triggers the TAS2R receptor and releases glucagon-like peptides-1 (GLP), which can help to reduce the blood glucose level. Moreover, Polyphenols found in medicinal herbs activate bitter taste receptors in the gut (Jang et al., 2007). Bitter taste receptors provide an essential link between alimentary chemosensation and metabolic disease. According to the Siddha pharmacology, phytopharmacological studies of the ingredients (Shenbagaraj et al., 2024), In-vitro analysis of the compound drug, CVC may synergistically act as a potent Antidiabetic agent in Non-Insulin Dependent Diabetes Mellitus (NIDDM) and Insulin Dependent Diabetes Mellitus (IDDM) by regularizing the insulin secretion and prevents the Diabetic complication.
Conclusion
Cuṇṭai Vaḷḷal Cūraṇam stands out as a promising drug in complementary therapy for managing T2DM, by its notable antioxidant, antidiabetic, and Antihyperlipidemic properties. Due to its taste, natural composition, and wide-range pharmacological efficacy, the therapeutic administration of CVC could be a drug of choice to inhibit the conversion from Pre-Diabetic to Diabetic. It also can be employed as an add-on drug among established Diabetes both in NIDDM and IDDM. Combining traditional Siddha medicine with contemporary scientific validation highlights the significance and potential of CVC in tackling modern health issues. Additionally, in-vitro assessment findings have not reflected the efficacy of CVC in T2DM for humans. Further in vivo studies and clinical trials are essential to substantiate these findings and fully assess CVC's safety and therapeutic potential.
Abbreviations
ABTS - 2,2′-Azino-Bis 3-ethylbenzothiazoline-6-sulfonic acid assay
AGE - Advanced Glycation End-products
CVC - Cuṇṭai Vaḷḷal Cūraṇam
DPPH - 2,2-Diphenyl-1-picryl hydrazyl assay
HMG-CoA - β-Hydroxy β-methylglutaryl-CoA
MRD - Medico Renal Disease
NFHS - National Family Health Survey
ROS - Reactive Oxygen Species
T2DM - Type 2 Diabetes mellitus
WHO - World Health Organisation
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author contributions
Conceptualization: SSR, SSP. Methodology: SSR, RV, SSP. Validation: SSR, AL, SR, PS. Formal analysis: SSR, RV, BV, AS, BS, ASB, SR. Writing - Original Draft: SSR, RV, BV, AS, BS, ASB. Writing - Review & Editing: SSR, RV, BV, AS, BS, ASB. Visualization: SSR, RV, BV, AS, BS, ASB. Supervision: SSR, AL, SR, PS.