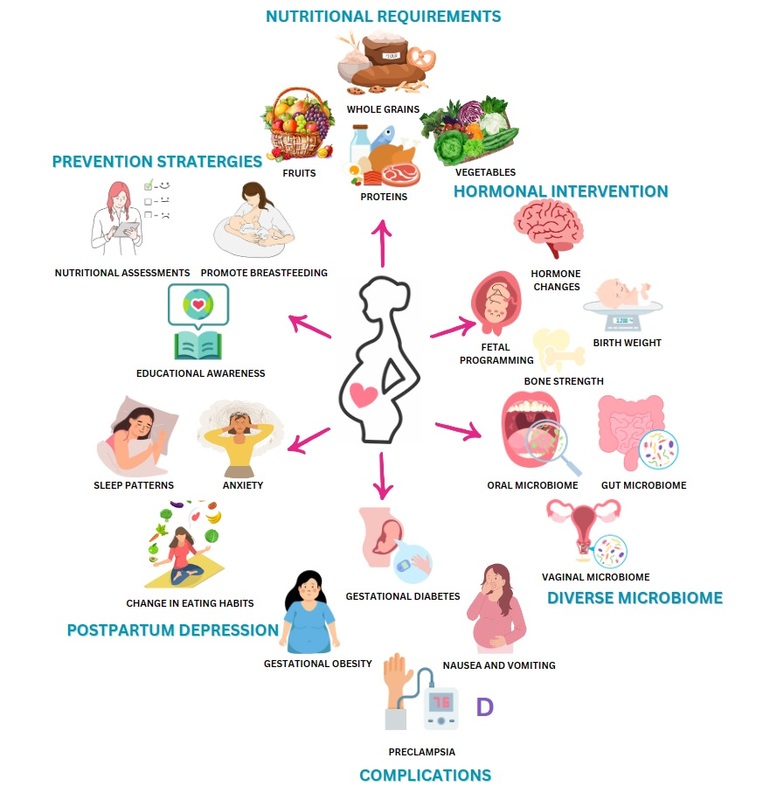
Introduction
The cellular discrepancy between the body's need for nutrients and energy and the available supply required for growth and maintenance, including the imbalance and excess in food consumption, is termed malnutrition. The significance of maintaining a regular, well-balanced diet is reiterated since it is critical in ensuring proper functioning, steady health, and body development throughout life. Survival, development of the body and mind, productivity and performance, health, and well-being are maintained well by balanced diet intake. Nevertheless, age, gender, and physiological changes like motherhood present distinct dietary needs. A woman's pregnancy is an important phase in her life, during which the growing fetus needs adequate nutrition. Malnutrition is a symptom that is impacted by several complex circumstances that affect the children's general health in the country. It is strongly linked to a poor diet and health issues in specific situations, like cultural beliefs and lack of access to necessary healthcare for individuals in low socioeconomic conditions, inappropriate childcare and feeding practices, a crisis in the home food supply, unhealthy housing, and a lack of education among parents, particularly among mothers. Pregnant women must ensure adequate nourishment to promote their own well-being and ensure a successful pregnancy outcome. The time when the body needs more macro- and micronutrients to meet its demands is crucial for pregnancy because of the increased nutritional needs. Thus, 53.8 million pregnant women worldwide suffer from vitamin A deficiency (VAD) and anaemia, two prevalent micronutrient deficiencies.
Intrauterine growth retardation (IUGR), low birth weight, and both mother and infant mortality and morbidity are frequently caused by poor nutrition during pregnancy when combined with infections. Since the impacts of malnutrition are pervasive and persistent, and prevention is the most significant course of action when eradicating it, it continues to rank among the world's most urgent health concerns. In addition to inadequate nutrition, psychological and social variables, mother’s comprehension of nutrition, and hormonal fluctuations that affect how they perceive their food choices while pregnant all have an impact on maternal malnutrition. A multitude of women experience impoverished micronutrient status, insufficient weight gain during pregnancy, and chronic energy deficiency. Maternal malnutrition is caused by low food intake, excessive energy expenditure, diets lacking in micronutrients, infections, and the demands of lactation and pregnancy (Figure 1). There exists a correlation between maternal undernutrition and increased rates of maternal morbidity and mortality, as well as an intensified likelihood of adverse pregnancy outcomes, including preterm birth and low birth weight. Subsequently, these consequences contribute to an increased likelihood of morbidity and mortality among neonates. According to the United Nations Children's Fund (UNICEF), lack of correct nutrition when pregnant accounts for 50% of maternal fatalities annually, and it is the main factor causing unfavourable pregnancy outcomes, being among the world's most pressing reproductive health issues. During prenatal consultations, emphasizing the significance of maternal nutrition as a vital component of a healthy pregnancy is vital. Adequate nutrition is crucial for maintaining the health of the mother throughout pregnancy and for the growth and development of the fetus; it can also have enduring consequences for the health of the mother and her child. A frequent term used to describe pregnancy is "teachable moment," when women are inspired to adopt healthier behaviours for their overall well-being. This is especially beneficial in light of the growing body of evidence showing that nutrition from infancy might influence an infant's chronic health (Developmental Origins of Health and Disease) (García, Ortega, Peral-Suárez, Bermejo, & Rodríguez-Rodríguez, 2020). Some of the most well-known examples include insufficiency of folic acid causing neural tube anomalies, alcohol usage during pregnancy leading to fetal alcohol spectrum disorders, and deficiency of vitamin D causing rickets (Seymour, Beck, & Conlon, 2019).
The majority of the 178 million children under five who are stunted (i.e., have a height-for-age Z score of less than −2) reside in South-central Asia and sub-Saharan Africa. Only 36 countries have a population of 160 million stunted children, which accounts for 90% of all stunted children worldwide and represents 46% of the total number of children residing in those countries. Out of the estimated 55 million children who are wasted, meaning they have a weight-for-height Z score below -2, around 19 million have severe acute malnutrition, which is indicated by a weight-for-height Z score of -3 or below together with edema, or severe wasting, which is indicated by a weight-for-height Z score below -3. Fetal undernutrition, also known as intrauterine growth restriction, is a widespread condition, particularly prevalent in south-central Asia (Black, Morris, & Bryce, 2003). There is a strong correlation between undernutrition and child mortality but there is less compelling evidence regarding the contribution of intrauterine growth restriction to the mortality of neonates and children under the age of five.
The fetus intrauterine environment affects the genetic control of fetal growth, even though the program contained in its genome drives fetal growth and development. The provision of nutrients and oxygen to mother is one element that is essential for the life and well-being of the fetus. The mother's physical size, composition, metabolism, and nutritional status—all developed during her own fetal life, childhood, and adolescence—determine her capacity to nourish her unborn child. The fetus can grow to its full potential if sufficient nourishment is available, which would lead to the delivery of a healthy infant that is the right size. The development of the fetus may be negatively impacted by any anomaly in the intrauterine environment. Fetal undernourishment results from the insufficient supply of nutrients to fulfill fetal needs, which can be affected by several aspects example maternal malnutrition, insufficient placental function, or increasing nutritional demand. It is also harmful to the fetus to receive more nutrients. An increased flow of nutrients, including glucose, from the placenta of a diabetic mother to the fetus can cause fetal enlargement or macrosomia.
In order to mitigate the likelihood of adverse consequences, such as gestational diabetes mellitus (GDM), pre-eclampsia, stillbirth, macrosomia, miscarriage, and caesarean section for the mother, as well as abnormal birth weight and an elevated susceptibility to childhood obesity, it is imperative for mothers to uphold a state of optimal health. Given the advantages this can have for the health of both the fetus and the mother, pregnant women have long been advised to abstain from alcohol and tobacco use in addition to engaging in regular physical exercise. The optimum outcome for both the mother and the newborn during pregnancy can be achieved by optimising the nutritional condition of the mother both before and throughout the pregnancy. Dietary recommendations for the best possible health during pregnancy have traditionally centred on eating a balanced diet and making sure that both macro- and micronutrient intakes are adequate. The way that woman's diets are viewed should centre on taking more thorough steps to safeguard and prepare the female population against triple nutritional and dietary issues. The early stages of life are a critical period of adaptability, extending from the moment of conception until around two years of age. During this period, developing cells and organs undergo modifications in response to environmental factors that impact the mother, such as stress, infections, alcohol consumption, smoking, chronic diseases, obesity, and malnutrition. These factors eventually dictate an individual vulnerability to a specific illness risk in adulthood. During gestation, the developing fetus is very susceptible to environmental factors, including energy balance and the availability of nourishment, which are crucial for optimal growth and development. Gestation not only involves increased nutritional and energy needs for the developing fetus, especially during the later stages of pregnancy, but also serves as a time of preparation for an even higher energy demand.
Considering that pregnancy is a period of heightened susceptibility to nutritional deficiencies and has a substantial impact on the growing fetus, it is essential to acknowledge the significance and complexity of nutrition during this period (García et al., 2020). Due to the fact that these conditions will fluctuate in response to the arduous demands of two years of breastfeeding and nine months of pregnancy, there is cause for concern regarding the nutritional status of pregnant women during gestation. This raises questions about the significance of nutrition for pregnant women throughout the pregestational, gestational, and breastfeeding periods (Castillo-Matamoros & Poveda, 2021). Essential components of balanced food during pregnancy incorporate eating foods that are high in macro- and micronutrients and energy, gaining the right amount of weight, following general and pregnancy-specific food safety guidelines, and abstaining from dangerous substances (Forbes, Graham, Berglund, & Bell, 2018). According to earlier research, Failure to adhere to these behaviours increases the likelihood of adverse pregnancy outcomes, including but not limited to preterm delivery, hypertension, low birth weight, and neurodevelopmental complications like fetal alcohol spectrum discord (García et al., 2020). The time between conception and the age of two is a formative one, during which the body undergoes remarkable plasticity. Cells and organs in development adjust to the mother's internal and external environments throughout this time in response to stress, infections, alcohol and tobacco use, chronic disease, obesity, and starvation. Whether an adult is at risk for a certain disease is ultimately determined by these criteria. Environmental factors, including as energy balance and nutrition availability, are especially important during gestation because they affect the fetus capacity to grow and develop normally. During the course of a pregnancy, the growing fetus has substantial nutritional and energy needs, especially in the later trimesters, and the body also prepares for even greater energy demands in the future (Castillo-Matamoros & Poveda, 2021).
During the first half of gestation, by utilizing increases in insulin sensitivity, the woman's body can adapt and store large amounts of energy as fatty acids and protein. During this stage of anabolism, insulin sensitivity changes are prominent. The next step, known as the catabolic phase, involves the mobilisation of maternal resources to ensure that the fetus receives enough nutrition through the placenta. In this early stage, micronutrients are also in high demand. Some examples of these elements are calcium, which is necessary for building bones and for intracellular communication, iron, which is necessary for building a number of proteins (including haemoglobin, which is vital for transporting oxygen and, by extension, for cellular respiration), and folic acid, which is essential for "one-carbon metabolism," the building block of the nucleic acids needed for cell replication (Mousa, Naqash, & Lim, 2019). Crucially, other intermediate vitamins and minerals are needed to facilitate metabolic processes in the biochemical pathways in which these three micronutrients engage. Since there is a strong correlation between poor pregnancy outcomes and neglecting nutritional care, nutritional care must be prioritized or integrated into core interventions during gestational care. This is because increased anabolic activity is largely responsible for the increased demands for nutrients during gestation (Herreño & M, 2016). As a result of the intensified physiological demands associated with human gestation, it is critical to maintain a healthy diet and nutrition throughout pregnancy to supply the energy reserves and macronutrient and micronutrient pools that support the numerous cell replication and differentiation reactions. This entails the suitable addition of the aforementioned three micronutrients. These ensure optimal fetal development and growth when combined with the physiological substrates. Obstetric complications that are significantly impacted by the nutritional status of the expectant mother and child, including intrauterine growth retardation, preterm birth, low birthweight, neonatal and maternal morbidity and mortality, and delivery complications, can be avoided with the aid of a nutritious diet (Castillo-Matamoros & Poveda, 2021).
Diet and Nutrition Supplementation During Pregnancy
Women must manage their diets before becoming pregnant, during pregnancy, and after giving birth. Instead of concentrating on specific nutrients or foods, dietary patterns are a developing field of study that encompasses the complete diet. Adhering to a nutritious diet prior to and throughout pregnancy has been associated with a reduced likelihood of developing pregnancy-related complications, including gestational diabetes mellitus (GDM), preterm birth, complications arising from obesity, preeclampsia, and gestational hypertension in specific instances. Particularly for expectant women who are obese, have undergone bariatric surgery, or have pre-existing diabetes mellitus (DM), nutrition therapy is the cornerstone of GDM treatment. A diet that promotes good perinatal outcomes and ensures a secure pregnancy is one that promotes a balanced intake of macronutrients. Considered healthful are diets that are abundant in fruits, vegetables, whole grains, seeds, legumes, seafood, monounsaturated fat-rich oils, and fibre, while being low in refined grains and fatty red meat. Additionally, processed foods, saturated and trans fats, and basic carbohydrates should be avoided in healthy diets (Marshall et al., 2022).
The extra energy needs of 300 kcal per day during pregnancy are classified as such. Calorie intake is not as crucial as nutritional quality. The amount of protein, vitamins, and minerals in food per 100 kcal is known as nutrient density. Many women, especially those in their teens, eat a diet high in low-density foods poor in nutrients. Pregnant women need 60 g of protein per day, which is around 15 g more than they would need otherwise. Certain vegetable oils and fish oils include omega-3 fatty acids, which are regarded to be vital for the development of brain and visual function in fetuses. Dairy-based vegetarian diets can offer a well-balanced food intake during pregnancy. As long as there is additional monitoring and counseling, as well as vitamin B12 and D intake along with some crucial amino acid supplements, vegan diets, which omit all animal products, are also safe to follow while pregnant. The US Public Health Service recommends that women of reproductive age take a daily dosage of folate (0.4 mg) before getting pregnant to reduce the risk of non-transformed disorders. To ensure enough iron intake during pregnancy, it is recommended to supplement with 30 mg of elemental iron daily, as diet alone may not be sufficient. Women following supplemental diets should incorporate 2 mg of copper and 15 mg of zinc, in addition to iron supplementation. The recommended daily intake of calcium during pregnancy is same to that outside of pregnancy: 1000 mg per day for women aged 19 to 50, and 1300 mg per day for those under the age of 18. Supplementing with calcium has not been associated with a decrease in hypertensive diseases or preeclampsia, according to randomised research (Bucher et al., 1997). Recent randomized controlled studies assessing the effectiveness of vitamin C and E supplementation in lowering preeclampsia rates have not shown a decrease in preeclampsia, growth restriction, or fetal/neonatal mortality rates.
Fiber, different minerals, vitamins A, C, and Bs, and other nutrients all rise, albeit to a lesser degree. Certain dietary guidelines do not become any better throughout pregnancy. Fluoride and vitamins D, E, and K remain unchanged. Using fluoride supplements during pregnancy is not recommended. However, it is important to advise the mother that bottled water cannot be relied upon as a supply of fluoride. While it is common for individuals to have inadequate calcium consumption before pregnancy. The Dietary Reference consumption (DRI) for calcium does not increase during pregnancy. Women in their childbearing years also exhibited inadequate consumption of vitamin E, magnesium, potassium, and fibre. This issue can be resolved by increasing the intake of fruits, vegetables, and whole grains. While not explicitly mentioned, it is assumed that the Dietary Reference Intakes (DRIs) for nutrients are higher in twin pregnancies. There are currently no set guidelines for prenatal weight growth recommendations or Dietary Reference Intakes (DRIs) for triplets and higher-order multiples; nevertheless, best practice information is accessible. Weight gain that occurs early (before 20-24 weeks) seems to be crucial. Eating properly becomes much more important with all the multiples because of the increasing requirements and even less room available. In conclusion, while calorie requirements rise, they do so at a slower rate than nutritional demands. The emphasis should be on smaller, more frequent meals and higher-nutrient foods, particularly as the pregnancy goes on and physical space becomes more of a concern. Patients should prioritise consuming whole grains instead of juice, opt for whole milk or yoghurt first (then transition to low-fat, followed by skim), favour red meats over white meats, select leaner cuts of meat, and opt for water instead of sports drinks. In order to maximise the nutritional value while minimising calorie intake, it is advisable to give preference to fresh or frozen fruits and vegetables, avoid fried meals, and restrict the consumption of additional fats and sugars. Women with elevated requirements, such as those carrying multiple fetuses, those with HIV, those who smoke, consume alcohol, or use drugs, or those who have a limited intake of animal products, are advised to take prenatal vitamins (multivitamin/multimineral). Some individuals utilise them as a form of protection rather than as a substitute for a nutritious diet (Cox & Phelan, 2008) (Table 1).
Current themes of concern include the consumption of liver and its potential hazards, the need of avoiding raw cheese, and the potential problems associated with consuming excessive amounts of tuna and other oil-rich seafood (beyond two meals per week). According to the National Institute of Health and Care Excellence (NICE), alcohol use should be severely limited during the remaining weeks of pregnancy since it may harm the fetus health during the first trimester of pregnancy (Aquino, Edge, & Smith, 2015). Moreover, alcohol is regarded as a concentrated source of energy, providing 7 kcal/g. As such, moderate drinking may raise energy intake and promote excessive weight gain during pregnancy. Maternal caffeine intake has drawn a lot of attention, as it is believed that higher intakes limit fetal development. Retrospective measurements of caffeine intake were made in one enormous prospective observational study conducted in two UK maternity units. The findings demonstrated that while small amounts of caffeine (up to 100 mg daily) are safe, larger amounts (over 200 mg daily) raise the risk of miscarriage, early delivery, and low birth weight in children. Two cups of tea or coffee provide about 200 milligrams of caffeine, but there are other rich sources as well, including caffeinated beverages. These beverages' decaffeinated equivalents could be worthwhile. Recognising the importance and intricacy of nutrition during pregnancy is crucial due to the heightened nutritional vulnerability of pregnant women compared to other stages of their lives. Additionally, pregnancy has a substantial impact on the future health and nutritional status of the fetus, which in turn affects the woman's health as an adult. Due to the physical demands of nine months of pregnancy and two years of breastfeeding, the nutritional needs of women during this period vary. Therefore, it is important to carefully consider the health and significance of nutrition for pregnant women throughout the stages of pre-pregnancy, pregnancy, and breastfeeding.
The Role of Essential Nutrients During Pregnancy
Pregnancy depends heavily on nutrition, which may also be a modifiable risk factor since it determines a person's lifetime risk of disease. The World Health Organization (WHO) has standards for prenatal care, but there are no thorough guidelines that describe the dietary requirements of women throughout the whole reproductive cycle, from conception to nursing and beyond. Considerable discourse has ensued regarding the criticality of sufficient and essential nutrition across the entire developmental continuum, encompassing preconception, pregnancy, delivery, infancy, and adolescence (Marshall et al., 2022).
Folic Acid
Folic acid is indispensable for the regulation and development of the genetic material within the body, which influences the synthesis of proteins and the expression of RNA. The demands increase during pregnancy as a result of heightened rates of cellular division, expansion, and DNA synthesis. Folic acid deficiency has been shown to be significantly correlated with neural tube defects (NTDs). NTDs occur during the fourth week of pregnancy, when the neural tube fails to completely enclose. Esophagogaly, encephalocele, and spina bifida are the three most prevalent NTDs. Folic acid, a synthetic form of folate, is found in fortified foods and dietary supplements. In comparison to natural folates, folic acid exhibits a bioavailability that is as much as twofold greater (Seymour et al., 2019). In addition to consuming nutritious foods, the US Public Health Service advises all women of reproductive age to consume 400 micrograms (0.4 mg) of synthetic folic acid daily. It is advised to increase the dosage to 600 mcg/d during pregnancy. In order to prevent a recurrence of NTD, the dosage is increased to 4 mg/d as a separate supplement from one month prior to conception through three months of pregnancy (to avoid excessive intakes of other nutrients). The potential of natural folate to mitigate the risk of non-traumatic diseases (NTDs) has not been substantiated, and its bioavailability is merely half that of synthetic folic acid, which is present in green leaves, legumes, and citrus fruits. Since 1998, the addition of folic acid to enriched wheat and grain products (140 mcg/100 gm) has increased consumption and reduced the risk of non-transformed diseases by 27%. Folic acid-fortified breakfast cereals, many of which contain 400 mcg per serving, are an alternative option for individuals who are unable or unwilling to consume folic acid tablets (Cox & Phelan, 2008). It has been demonstrated that taking folic acid pills empty-handed increases the nutrient absorption. It is advised that pregnant women who are at risk for NTDs take 5 mg of folic acid daily. A woman is deemed to be susceptible to having a neural tube defect (NTD) if she has previously experienced a pregnancy impacted by an NTD, has a partner with a familial predisposition to NTDs, is using medicine that could impede the metabolism of folic acid, is taking insulin for diabetes, or possesses any combination of these factors. Medications used to treat infertility, acne, anti-epileptic treatments, and insulin can all interfere with the metabolism of folic acid. Vitamin B12 insufficiency can be concealed by high amounts of folic acid supplementation; therefore, it is critical to assess a woman's vitamin B12 status before prescribing high-dose supplementation, especially for those who are more vulnerable to B12 deficiency, such as vegetarians and vegans. Folic acid supplements are needed since it is hard to get the appropriate levels from diet alone (Seymour et al., 2019).
Omega-3-Fatty Acids
The specific functions of omega-3 fatty acids and long-chain polyunsaturated fatty acids have received significant study in recent years. Due to its influence on the development of the retina and brain, docosahexaenoic acid C22:6n-3 (DHA) has been the subject of a great deal of research on pregnancy and breastfeeding (De Luca, 2013). Preterm labour and the avoidance of pre-eclampsia are two pregnancy outcome indicators for which omega-3 fatty acids have not been proven. The precursor alpha-linolenic acid (ALA) increases marginally but is poorly converted to docosahexaenoic acid (DAA); it has not been shown that increasing DHA levels in the mother, fetus, or breast milk will result (Luca, 2013). Foods touted as having just omega-3s definitely include ALA, most likely from flax (canola and walnuts are other good sources of ALA), therefore they should not be trusted. DHA is essential for brain development, and greater baby DHA levels are linked to improved neurological and visual test results (Luca, 2013). However, whether early exposure to DHA and eicosapentaenoic acid, two n-3 fatty acids might benefit a child's development in the long run is unknown. Supplements for expectant and nursing mothers have not always been shown to be beneficial in studies (Luca, 2013) . Fatty fish, along with brains and certain fortified eggs, are the richest sources of DHA; it is advised to eat 1-2 servings of fish per week. Salmon, herring, trout, anchovies, mackerel (not King mackerel), and sardines are among the fish with the highest DHA content and the lowest mercury content.
Calcium
Strong bones and teeth need calcium for development and upkeep. In addition to being crucial for maintaining ideal bone health, calcium may guard against pregnancy-related hypertension diseases such as pre-eclampsia (Seymour et al., 2019). The daily recommended intake (DRI) of calcium for non-pregnant women is 1000 mg for those aged 19–50 and 1300 mg for those aged 18–49. Consuming more than what is suggested does not enhance breastfeeding, improve pregnancy outcomes, or prevent the inevitable bone loss that occurs during pregnancy and maybe during lactation (Adams, Miller, Agbenyo, Ehla, & Clinton, 2023). Proudly promote milk and yoghurt as the best calcium sources. Using skim milk instead of cheddar cheese allows a woman to get the same amount of calcium with half the fat calories. Calcium retention is reduced by cheese and cottage cheese due to their high protein and salt to calcium ratios. It would take more than eight cups of cooked spinach to equal the calcium in one cup of milk. In order for soy milk to provide the same amount of calcium as one cup of milk, it needs to include half of the USRDA, which is 500 mg of calcium per cup. Such a level of fortification is unusual. The calcium absorption rate of fruit juices is decent, but a lot of goods leave a sludgey residue at the bottom that won't budge no matter how much shaking. A number of ready-to-eat breakfast cereals have been calcium enriched to a high degree. Women who are already low-calcium consumers may benefit from increasing their calcium intake for the sake of their babies' blood pressure (Brion, Leary, Lawlor, Smith, & Ness, 2008). However, there is no correlation between maternal nutrition and blood pressure in children, according to multiple research projects. Supplemental calcium reduces the occurrence of pre-eclampsia in hypertensive disease-prone women whose diets are deficient in this mineral, according to Cochrane reviews.
Vitamin D
Prenatal vitamin D is essential for healthy bone development in the unborn child. Vitamin D regulates blood calcium levels by affecting the small intestine absorption of calcium and phosphorus as well as losses from bone. A vitamin D deficiency increases the chance of developing infantile rickets and can have detrimental effects on fetal growth and bone formation. When a person has rickets, their bones become weaker and more pliable, which can lead to abnormalities. Humans obtain their recommended daily intake of vitamin D from their diets in the forms of cholecalciferol and ergocalciferol, or via UV light exposure (sunlight), converting precursors in the skin to cholecalciferol. Sunlight exposure and its conversion, however, vary greatly across people and are influenced by factors including season, skin tone, and skin exposure. Because the UV intensity of sunlight is substantially lower in the winter, it is crucial for women who do not receive enough sun exposure throughout the year to consume enough vitamin D in their diet. It is recommended that all citizens of the United Kingdom consume a dietary supplement every day comprising 10 milligrams of vitamin D. This advice is especially crucial in the winter, when there is less sun exposure, and for expectant mothers who may have a reduced vitamin D balance. Oily fish (herring, salmon, and mackerel), eggs, and vitamin D-fortified foods, such as certain margarine and spreads, are dietary sources of vitamin D (Seymour et al., 2019). The increasing reliance of adolescents on vitamin D for calcium homeostasis and the detrimental effects of inadequate sun exposure or obesity-related vitamin D deficiency on bone mineral density are being acknowledged as significant public health concerns. Experimental vitamin D deficiency is prevented from promoting the development of autoimmune disorders, such as inflammatory bowel disease, by the active form of vitamin D. This observation has led to the hypothesis that vitamin D, an environmental component, exerts an influence on the development of immune-mediated disorders (Cantorna, Yu, & Bruce, 2008). The differential susceptibility of children to acute lower respiratory infections and M. tuberculosis infection. It seems to be regulated by variants of the vitamin D receptor allele. The activation of the TLR by human macrophages and the upregulation of the vitamin D receptor and vitamin D-1-hydroxylase genes are the mechanisms by which a mycobacterial infection induces intracellular death of M. tuberculosis and the production of the antimicrobial peptide cathelicidin.
Iron
Red blood cell growth, particularly the production of haemoglobin, depends critically on iron. It is also necessary for the body to function with other enzymes, support the immune system, synthesise components of connective tissue, and form neurotransmitters as a cofactor (Seymour et al., 2019). The iron needs of pregnant women nearly triple and are improbable to be met through dietary sources. Consequences of iron deficiency during pregnancy include adverse effects on the cognitive and behavioural development of the expectant child, preterm birth, low birth weight, and perinatal mortality (Seymour et al., 2019). Eating meals high in iron is the best approach to reaching the necessary daily intake of 14.8 mg. Overconsumption of iron can lead to adverse consequences in mothers, such as nausea, vomiting, heartburn, diarrhoea, and stomach discomfort. Additionally, it may impede the assimilation of minerals such as copper and zinc. Iron deficiency was estimated to affect 12% of women aged 20 to 49 and 16% of women aged 16 to 19 among women of reproductive age between 1999 and 2000. Women of colour (22 percent Mexican American and 19 percent non-Hispanic Black) had higher rates than non-Hispanic White women (10 percent). Postpartum anaemia is more prevalent among pregnant women who did not consume iron supplements (Bodnar, Cogswell, & Scanlon, 2002) and low-income women, particularly those from minority groups, are less likely to take iron-containing supplements (Cogswell, Kettel-Khan, & Ramakrishnan, 2003). The CDC provides cut points for normal haematocrit and haemoglobin readings, which fall during the second trimester due to haemodilution. Iron deficiency anaemia is often diagnosed by a lower-than-normal haematocrit or haemoglobin level, and it is assumed to be treated. Short interconnectional periods put women at a higher risk of iron deficiency anaemia in the ensuing pregnancy. It has been demonstrated that giving nonanemic women 30 mg/d of iron between weeks 20 and 28 improves birth weight and reduces the incidence of live births and premature live births while maintaining anaemia rates. This may be because the fetus and placenta get iron preferentially. Using high-iron dietary sources may yield a far better response in the treatment of iron deficiency anaemia than just depending on iron supplementation. Haem and non-haem iron are the two forms found in food. Meats, poultry, fish, and shellfish all contain substantial iron, of which 20–30% is absorbed. These foods include non-hem iron as well; only about 5% of it is absorbed. Other sources of non-heme iron include nuts, seeds, legumes, fortified food items (such as some morning cereals), and green leafy vegetables (Kim, Sitarik, Woodcroft, Johnson, & Zoratti, 2019; Malik et al., 2022). Pregnant women who eat vegetarian should have their iron levels checked often since they are more susceptible to iron insufficiency than other diets. Pregnant women can optimise their iron absorption by being advised of dietary components that can either enhance or reduce their absorption of non-hem iron (Seymour et al., 2019). Certain polyphenols, such as those in tea and coffee, have been demonstrated to decrease iron absorption, whereas vitamin C boosts it. Enhancing iron assimilation can be easily achieved by combining meals containing iron with vitamin C-rich foods (e.g., citrus fruits, peppers, and tomatoes) and by refraining from consuming tea and coffee during meals.
Vitamin B12
Both neurological and blood health need to take vitamin B12. The 1.5 mg per day advised for non-pregnant women and the recommended dietary intake for pregnant women are the same. However, because the fetal supply depends on food intake which is advantageously transferred by the placenta—regardless of maternal storage, vitamin B12 consumption during pregnancy is especially crucial. Vitamin B12 is derived exclusively from animal products, including meat, poultry, fish, shellfish, eggs, and dairy products, due to the fact that the microbes required for its synthesis are exclusively present in these items. Generally, pregnant women who follow a vegan or vegetarian diet consume insufficient amounts of vitamin B12. For vegetarian women to meet the daily requirements for vitamin B12, they must make sure they are drinking enough milk and dairy products. Vitamin B12 supplements are recommended for women who follow a vegan diet or opt to cut out all animal products from their meals, such as dairy, eggs, and milk (Seymour et al., 2019).
Iodine
The generation of thyroid hormones in a healthy state requires the element iodine. Iodine shortage can cause issues with the synthesis of thyroid hormones in both the mother and the fetus, which can impact the fetus growth and development as well as the infant's mental health. In addition to being the recommended daily allowance for non-pregnant women, 140 mg of iodine is also advised for expectant women. Seaweed, fish, seafood, eggs, low-fat dairy products, and iodized salt are among the foods that provide iodine to the body. Although kelp and seaweed pills have a high iodine content, they should not be used when pregnant because of the possibility of hazardous iodine levels (Seymour et al., 2019).
Vitamin A
Retinol, as well as precursors to vitamin A such as carotenoids, are dietary sources of the fat-soluble vitamin A. Eye health, fetal development, and the immune system are all enhanced by the mother's vitamin A intake. However, too much vitamin A can be teratogenic, meaning it increases the fetus chance of birth abnormalities. Supplements containing more than 700 mg of vitamin A should not be taken by pregnant women unless they are deficient in the vitamin or have received a different recommendation from a licensed healthcare provider. To prevent consuming too much vitamin A when pregnant, it is also advised that women abstain from consuming liver or liver-derived products like pate or fish liver oils (Seymour et al., 2019). The potential advantages of administering vitamin A supplements in the initial days after delivery to infants in cultures with a prevalent deficiency in this nutrient have been shown to diminish premature infant mortality caused by infections. When combined with zinc supplementation, vitamin A enhanced the neonatal population's immunological response to the hepatitis B vaccination and reduced the probability of fever and clinical episodes of malaria in Africa. Inducing the gut-homing specificity of T cells, the vitamin A metabolite retinoic acid (RA) enhances the expression of integrin alpha4beta7 in mothers who are positive relative to healthy infants (Cunningham-Rundles, Ahrn, Abuav-Nussbaum, & Dnistrian, 2002). Vitamin A deficiency is indicated by a low level of retinol in the mother's serum; this deficiency is a risk factor for transmission from mother to child. Increasing the vitamin A intake of HIV-positive infants and postpartum mothers improves their chances of survival. Nevertheless, in the case of breastfed infants who were initially negative for HIV but subsequently acquired the virus through breast milk, the exact supplementation approach negatively impacted their survival rate. Subsequent investigations have demonstrated that mannose-binding lectin (MBL) gene polymorphisms govern the HIV infection response to vitamin A. Component of the innate immune system, MBL binds to carbohydrate ligands on the surface of numerous pathogens and activates the lectin pathway of the complement system. Individuals harbouring variants of the MBL-2 gene demonstrate compromised innate immunity, rendering them more susceptible to contracting HIV. Neonatal evaluation of supplementation with beta-carotene and vitamin A. Infants with MBL-2 variants in the control group exhibited higher rates of HIV transmission from their mothers in comparison to those in the supplementation group, according to a supplementation study. Overall, the supplementation studies demonstrate that giving HIV-positive children selected vitamin A supplements increases their survival; however, the trials do not offer sufficient data to support the recommendation that pregnant women living with HIV should also take vitamin A supplements.
Vitamin E
Strong antioxidant vitamin E improves the response mediated by macrophages and monocytes. Recent research indicates that vitamin E treatment for atopic dermatitis participants results in decreased blood levels of immunoglobulin E and improved eczema. As previously mentioned, recent research indicates that a vitamin E deficit may increase the aggressiveness of viral infections by altering the virus itself (Beck, Handy, & Levander, 2004).
Zinc
Worldwide, a prevalent issue is gestational zinc insufficiency, which is brought on by an imbalance between intake and increasing need. The immune system and neurobehavioral development of new-borns were enhanced by the pregnant mother's zinc supplementation. Experimental models of zinc deficiency have provided evidence that "sequestration-induced deficiency" can induce teratogenic effects on fetal development in individuals with equivocal zinc status or illnesses. Additionally, fetal loss or malformation was more prevalent among mothers without adverse events who consumed a poor diet. Pregnant women who were afflicted with acrodermatitis enteropathy (AE), a genetic zinc deficiency characterised by an autosomal recessive defect in zinc absorption, consumed insufficient compensatory zinc. Circulating zinc levels decline during pregnancy due to haemodilution, reduced levels of zinc-binding protein, hormonal changes, and active transfer of zinc from the mother to the fetus. While accurately determining zinc levels can be difficult, it is generally accepted that deficiencies occur frequently during pregnancy, particularly in women who consume an all-vegetarian diet rich in dietary phytate, an inhibitor of zinc absorption. Mother's milk can provide zinc to a newborn. According to a recent study, in healthy newborns, greater levels of the CD4 T-cell receptor expression were connected with higher levels of zinc and thymulin. While deficiencies in the mother's capacity to transfer zinc into milk may contribute to an infant's zinc insufficiency, preterm newborns are more likely to require zinc and are less able to store it. Acute dermatitis or hyperkeratotic plaques are the skin lesions that appear in infants with AE, together with diarrhoea, baldness, and an increased risk of infections brought on by a severe immune deficit. Immune deficiencies include skin test anaesthesia, lack of NK cell function, severe thymic atrophy, and extensive lymphopenia. When sufficient zinc is supplemented, all symptoms go away. Zinc-enriched formulas improved delayed-type hypersensitivity as seen by increased salivary IgA, accelerated lymphoproliferative responses, and better skin test reactivity in babies with protein-calorie deficiency. They also improved linear growth in these children (Cunningham-Rundles et al., 2009).
Vitamin C
Through early rupture of membranes, dietary vitamin C consumption is directly associated with preterm delivery by increasing the likelihood of spontaneous preterm birth. Additionally, genetic variations in vitamin C transporters may play a role. As a powerful antioxidant and free radical scavenger, vitamin C is beneficial. During infections and stress, the concentration of vitamin C in leukocytes and plasma decreases. Compared to penicillin alone, antioxidant vitamin supplements, such as vitamin C, have been demonstrated to enhance children's immunological response to group A streptococcal infection (Ahmed, Zaman, & Ali, 2004). Supplementation may increase levels of the antioxidant plasma glutathione, which may improve phagocytosis and NK cell function. Reduced stomach juice ascorbic acid content is linked to Helicobacter pylori infection, and this impact is more pronounced in children whose strain A is CagA-positive. H. pylori is susceptible to the antibacterial actions of vitamin C. Household contact can expose children to H. pylori infection, which is linked to a lifetime risk of stomach cancer and an increased prevalence of reflux disease in infancy.
Selenium
Selenium exhibits an independent antioxidant effect when transcription and expression of manganese superoxide dismutase and uncoupling protein are diminished. This property is linked to selenium vital function in the antioxidant enzyme glutathione peroxidase. Selenoproteins have a significant impact on leukocyte and NK cell activity as an antioxidant component of the host defense system. By preventing some agonists, such as LPS, from activating NF-kappa B, selenium influences TLR signalling. In this context, deficiency is linked to congestive heart failure and might be a crucial aspect of protein-calorie malnutrition. Selenium deficiency and enteroviral infection are two factors in juvenile cardiomyopathy (Keshan). The virulence of two RNA viruses, coxsackie B and influenza, can be increased by deficiency in selenium and vitamin E through variant selection and perhaps direct impacts on viral phenotypic. This might have significant consequences for human transmission. Selenium insufficiency may, under some conditions, provide protection against influenza A, according to one experimental research. Selenium may offer defence against some viral infections that cause cell death. Without having an immediate impact on HIV progression, selenium supplementation improves child survival in HIV infection. Nonetheless, it has been shown that maternal selenium supplementation enhances HIV viral shedding and may raise the risk of transmission from mother to child.
Table 1
Nutrients and their benefits in different trimesters during pregnancy.
Gut microbiome
The adult human intestine harbours an estimated 15,000 to 36,000 species of bacteria, representing more than 1800 genera. A bacterial classification is utilised to classify 94–98% of the isolated microorganisms: Actinobacteria (3%), Firmicutes (64%), Bacteroidetes (23%), and Proteobacteria (8%). The remaining 2% of taxa, while less prevalent, comprise an exceptionally heterogeneous assortment, thereby augmenting the intricacy of the gastrointestinal microbiota (Frank et al., 2007). Women tended to have a greater proportion of Proteobacteria strains, which are associated with inflammation, in their intestinal microflora during the third trimester of pregnancy. During the third trimester, there is an increase in the population of Bifidobacteria, Proteobacteria, and lactic acid-producing bacteria, and a decrease in the population of butyrate-producing bacteria. There is a substantial increase in the total quantity of bacteria and notable changes in the composition of the gastrointestinal microbiota during pregnancy, especially in the latter stages. An increase in the abundance of Actinobacteria and Proteobacteria phyla strains and a reduction in the diversity of individual bacteria characterise this evolution (Table 2).
Dietary fiber is crucial in establishing the human microbiome, as are lipid and protein sources that supply essential amino acids. Insufficiency of upper gastrointestinal tract enzymes that catalyse carbohydrate metabolism dictates the fermentation processes that occur in the lower intestine (Vojinovic et al., 2019). It has been demonstrated that pregnancy affects the mother's gut microbiome, which varies in the first and third trimesters. Carbohydrates from dietary fiber travel undigested through the upper gastrointestinal tract and into the large intestine, during which the anaerobic microorganisms undergo fermentation. Intestinal microbiota during the initial trimester of pregnancy resembles that of non-pregnant, healthy individuals. Nevertheless, a substantial transformation occurs in the intestinal microflora composition between the initial and third trimesters (Table 4; Table 3). Studies involving rodents incubated with intestinal microflora from pregnant women during the third trimester of gestation observed an increase in body weight and insulin resistance, which mirrored the diabetogenic changes observed in pregnant women. The aforementioned findings could suggest that modifications to the gut microbiota during gestation play a role in developing pregnancy-specific metabolic alterations, including elevated levels of inflammatory markers and energy content. Intestinal microflora increases adipocyte-stimulating factor secretion during fasting, stimulates catabolic reactions, and strengthens the immune system; these functions may contribute to the way in which the microflora influences the weight gain of expectant hosts. Long-term detrimental consequences for the host organism and its offspring may result from dysbiosis, which refers to the continued alteration of various factor compositions induced by the microbiota. Such consequences may include obesity, enteritis, diabetes, and metabolic syndrome. Microflora in the human intestine may influence the regulation of nutrient metabolism, and dysbiosis may be associated with an increased uptake of energy resources.
Along the gut-brain axis, which connects the central, enteric, and autonomic nervous systems, bidirectional communication occurs via the hypothalamic-pituitary-adrenal (HPA) axis. The microbiota-gut-brain axis is comprised of gut microorganisms (including bacteria, viruses, fungi, and archaea), as well as their metabolites and by-products. These components also contribute to this bidirectional communication. The vagus nerve, which connects the brain to the abdomen and controls internal organ functions such as respiration, digestion, and pulse rate, is the tenth cranial nerve. Comprised of efferent and afferent neurons, it facilitates communication between the brain and various organs, including the intestines, where the impact of the gastrointestinal microbiota can be felt. The brain is thereby able to sense the gastrointestinal environment via this physical connection. A growing body of research suggests that there is a reciprocal relationship between the immune system and gut-brain signalling, demonstrating an interdependence (Salvo-Romero, Stokes, & Gareau, 2020). Low-grade systemic inflammation is linked to a variety of neurological disorders, including autism spectrum disorders, Alzheimer's disease, Parkinson's disease, epilepsy, and cerebrovascular diseases. This suggests that the immune system is compromised and that the gastrointestinal microbiota is unbalanced. Research on germ-free mice and mice that were administered broad-spectrum antibiotics has demonstrated that the gut microbiota has an impact on intestinal immunity, thereby influencing both systemic and local immune reactions within the organism (Fung, 2020). Recent studies have established a correlation between gut hormones and the modulation of mood, anxiety, depression, and obesity. Moreover, the gut microbiota may have an impact on these hormones and mood maintenance (Sun, Li, & Nie, 2020). An increasing body of evidence suggests that the gut microbiota and neuroendocrine system, both of which are linked to conditions such as irritable bowel syndrome and depression, engage in bidirectional communication. The hypothalamic-pituitary-adrenal (HPA) axis, which regulates mood, stress response, and immune function, can be stimulated by the intestinal microbiota (Bao & Swaab, 2019). Additionally, neurotransmitters like dopamine and serotonin, which have hormonal properties, may be influenced by the gut microbiota (Figure 2).
The intestinal mucosa interacts with microorganisms along with micro- and macronutrients that act as substrates for host and microbe cells. As a result, hormonal cascade and signal transduction is triggered, leading to absorption and transport to other body tissues. Various pathways digest nutrients in the gut; they may either be immediately broken down by the host cells like fatty acids, which activate receptors that produce hormones like GLP1, promoting insulin release. They may also be the metabolic products of the microbes, leading to the release of secondary molecules that regulate important body processes. For instance, insoluble dietary fibres fermented to acetate (for appetite regulation) (Silva, Bernardi, & Frozza, 2020), butyrate (for memory, mood, cognition) and propionate (preventing diabetes mellitus, obesity and stress) short-chain fatty acids (SCFAs) (Silva et al., 2020). After being absorbed in the intestine, they modulate the CNS by crossing the blood-brain barrier, while supporting its integrity and prevent leakage, whose depletion can adversely lead to Alzheimer’s (Dinan & F, 2017; Ratsika, Codagnone, O’mahony, Stanton, & Cryan, 2021). Dendritic and T-cell functioning is regulated, boosting the immune system and inhibiting cytokine production. These directly impact early fetal brain development and neurulation, mediated by nutrient-microbiota crosstalk (Mcdonald & Mccoy, 2019). Several nutrients and microbial metabolites, such as B complex vitamins, SCFAs, and polyphenols, have epigenetic potential and can influence fetal programming during sensitive developmental periods. Developmentally sensitive times are crucial windows of opportunity where changes may cause permanent adjustments to the way the body grows and functions. Preconception, pregnancy, and the perinatal and early postnatal phases are among them (Robertson, R, B, & J, 2019). Neuronal, immune, endocrine, and metabolic processes mature at a rapid rate during this period. Weight gain, metabolism regulation, brain development, microbiota composition, and immune system priming—all of which have a lifelong impact on an individual's health—are all significantly influenced by the type and quantity of food ingested during these periods (Huus et al., 2020). In order to mitigate the risk of neural tube defects and other malformations, it is recommended that expectant or intending women incorporate micronutrients, including folate, into their diet. Nevertheless, several variables, including vitamin kinetics, drug-microbiota-nutrient interactions, and drug use, may impact the availability of folate and raise the possibility of congenital abnormalities. According to research, factors in the environment that affect parents' health can affect their children's behaviour in later life. Fathers' immune system activation from infection, for instance, may have an impact on the behaviour of their offspring for at least two generations through the epigenetic regulation of reproductive cells (Tyebji, Hannan, & Tonkin, 2020). Environmental factors can exert an impact on the maternal immune system both prior to and throughout pregnancy, potentially resulting in enduring modifications to the well-being of her children from infancy to maturity. During pregnancy, the brain undergoes significant structural and functional changes, including axonal growth, synapse formation, and neuronal connections. These changes are essential for later-life cognitive development and behaviour. Brain function may be negatively impacted for some time after disturbances during these time-specific events. In addition to environmental factors including smoking, air pollution, infections, and radiation exposure, maternal factors including nutrition, lifestyle, mental health, and antibiotic use can influence the development of the fetus and the health of the expectant mother. Prenatal nutrition by the mother can influence structural and functional changes in the brain of the progeny during the perinatal period. The impact of nutrient intake on neurotransmitter pathways, synaptic transmission, and signal-transduction pathways ultimately determines the structural characteristics of the brain at both the microscopic and macroscopic levels Hung et al., 2021. For instance, by controlling hippocampus BDNF in rodents, omega-3 polyunsaturated fatty acids (PUFAs) are known to influence brain plasticity, cognition, and health. Omega-3 fatty acid intake during the formative years is linked to better cognition in humans, while lower levels are linked to mental health issues and neurodegenerative diseases. Maternal malnutrition whilst pregnant may cause abnormal neurodevelopment in the fetus, which may result in related problems in the adult offspring. The development of the nervous system in progeny may be influenced epigenetically by molecules associated with one-carbon metabolism, such as betaine, choline, and folate (Ars et al., 2019). Obtaining these nutrients is beneficial when consuming green leafy vegetables, beets, wheat, or seafood (Craig, 2004). Folate can be produced by specific gastrointestinal bacteria as well. A macronutrient and micronutrient profile that is optimally nourished is necessary for the brain to operate at its optimum capacity (Table 5). The microbiota composition of the progeny may be influenced, potentially impacting social behaviour and overall health, according to preclinical investigations conducted on rodents utilising maternal dietary manipulations (e.g., high-fat or high-fiber diets) during pregnancy (Buffington et al., 2016).
Table 2
Theprevalence of microbiota in gut and oral sites during gestation.
Table 3
Theplacental microbiota observed during pregnancy
Table 4
The change in vaginal microbiota during each trimester.
Hormonal intervention
Gut microorganisms produce neurotransmitters such as gamma-aminobutyric acid (GABA), serotonin, dopamine, noradrenaline, and tyramine, in addition to the neuroactive amino acids tyramine and tryptophan and short chain fatty acids (SCFAs) (Chakrabarti et al., 2022). Mood, thought, sleep, and appetite regulation are all influenced by serotonin. Since they raise the amount of serotonin that is available in the brain, selective serotonin reuptake inhibitors (SSRIs) are frequently prescribed therapies for depressive symptoms. Research also emphasizes that tryptophan is the only amino acid that functions as serotonin precursor. It has been suggested that the gut microbiota may affect the absorption of tryptophan and, consequently, the synthesis of serotonin. In the enteric system, 90% of total tryptophan is produced via the kynurenine pathway, which also generates kynurenic and quinolinic acids that are essential for the operation of the enteric and central nervous systems, respectively (Gheorghe et al., 2019). A study on fetal mice by Xin Ye et al. depicted that perturbed maternal diet affects the fetal brain, predisposing to postnatal autism spectrum disorders. Serotonin and oxytocin which are vital in the development of neural connectivity, are fuelled by glucose transporters GLUT3, whose sufficiency is crucial (Ye et al., 2021). Beyond the peripartum period, oxytocin is also required to induce labour and for a healthy, and safe delivery (Monks & Palanisamy, 2021). Dopamine, a crucial neurotransmitter involved in the reward systems of the brain, functions as a norepinephrine initiator - which influences behaviour and cognition, and adrenaline- which is also known as arousal and alertness. Parkinson's disease, schizophrenia, and addiction are disorders linked to dopamine deficiency, which are either synthesized or metabolized by certain bacteria (Rekdal et al., 2020). GABA, synthesized mainly by Bifidobacterium and Lactobacilli, significantly impacts cognition, behavior, and the body’s response to anxiety, fear, and stress (Jewett & Sharma, 2020). A deficit, however, causes depression, autism, and schizophrenia.
Miscarriage
A miscarriage occurs when a pregnancy unexpectedly ends between the twelfth and twenty-fourth week of gestation, which is recorded to occur in at least 1 of 5 pregnancies. With significant physical and emotional effects on the individual, it is additionally linked to substantial healthcare expenses. Nearly 50% of the cases are attributed to chromosomal aberrations, nevertheless, there is further evidence linking substantially higher rates of miscarriage to factors involving ethnic origin, the mother's psychological state, extremely high or low BMI pre-pregnancy, stress, smoking, use of non-steroidal anti-inflammatory drugs, and alcohol consumption. In a study conducted in Pakistan, it was found that 23% of the population that suffered a miscarriage were having a poor diet, while 20% was due to hypertension, and 6% due to depression. 8% of the population miscarried having a history of C-section due to improper or incomplete healing or uterine involution. In another research as well, it was found that alongside socioeconomic factors and poverty, malnutrition was a driving force in occurrences of miscarriage, abortion and preterm labour (Malik et al., 2022). Micronutrients with antioxidants such as Vitamin A, B9, E, C, and iron have been suggested as interventions in severe cases of recurrent miscarriages, to normalise metabolism and defend a normal pregnancy (Coomarasamy et al., 2021).
Supplementation and Dietary Modification
In certain cases, a prolonged administration of broad-spectrum antibiotics to treat infections, end up depleting the enteric flora, which can impair the immunity and digestion in neonates and new mothers. Severe consequences like diminished absorption of nutrients, synthesis of vitamins and non-essential amino acids, and accelerated risk of obesity, asthma, diabetes, and infections. The simplest solution to this would be an oral supplementation of prebiotics and probiotics, which either stimulate the growth of, or supply living microbes to the system which confer good health. They compete to adhere to the mucosal epithelium, thereby strengthening the barrier, increasing its IgA and antimicrobial substance turnover to improve immunity. Pro-inflammatory pathways are downregulated and anti-inflammatory cytokines are produced. These protect against obstetric and paediatric disorders, including obesity, hypersensitivity, gastrointestinal complications, and upper-respiratory difficulties. Studies have also found that polyphenols, omega-3 fatty acids (Swanson et al., 2020), inulin, fructo- and galacto-oligosaccharides can be used as prebiotics (Quigley, 2019). Probiotics include Lactobacillus spp., Bifidobacterium spp., Streptococcus thermophiles etc (Quigley, 2019).
The ancient proverb goes something like, "let food be thy medicine, and not medicine thy food." In a nut, it emphasises on the fact that a good diet can indeed preserve the body's equilibrium and health, as well as stop or lessen the onset of certain illnesses (Zhuang, H, S, J, & Q, 2019). The microorganisms that inhabit the gut, especially of fetus and neonates perform a multitude of vital roles, including but not limited to immune system development and function and vitamin B and K synthesis. A healthy titre of native bacteria possesses the capability to compete against pathogenic organisms wanting to colonise the gastrointestinal tract and also affect the metabolism and functioning (Ansaldo, Farley, & Belkaid, 2021). Gestational age, prenatal factors, genetics, method of delivery, maternal health and nutrition, and postpartum feeding regimen are all influential in determining the composition and development of the intestinal microbiota in the progeny.
Table 5
The effect of various food and sources on microbiota.
Fetal programming
British physician and epidemiologist David Barker created the concept nutritional programming, sometimes known as the ‘Barker hypothesis’. It holds that critical fetal development phases determine metabolic and hormonal changes that may affect health later in life. According to fetal programming (Parrettini, Caroli, & Torlone, 2020), unfavourable factors such maternal starvation during early organogenesis can permanently change organ and organ system anatomy. Epidemiological causes include environmental triggers including dietary excess or deficiency and chronic structural, metabolic, and functional organ damage in children (Alemany, 2024). It is linked to ‘civilization diseases’ such type 2 diabetes (T2D), hyperlipidemia, obesity, osteoporosis, high blood pressure, cancer, and cardiovascular disease (Stolarczyk, 2022). Maternal body composition and malnutrition-related energy extremes (ingestion and assimilation) usually affect fetal life (Ma et al., 2020).
Up to 70% of adult gut microbiota is stable and identical to that of a child's first three years, but the rest can be affected by nutrition, lifestyle, pet exposure, and antibiotics. Proper nutrition is important throughout life, including during pregnancy (Kim et al., 2019). Recent investigations have found maternal bacteria in umbilical cord blood, placenta, amniotic fluid, membranes, and newborn meconium, which may have entered via maternal circulation (Raspini et al., 2020). The mother's microbiome changes during gestation, post-partum weight, GDM prevalence, nutrition before and during conception, and intergenerational antibiotic use (Chu, Valentine, Seferovic, & Aagaard, 2019).
Effects of malnutrition
Enhancing a woman's dietary habits and general well-being prior to, during, and subsequent to pregnancy may potentially yield favourable results for obstetrical operations, perinatal survival, fetal development, and even the long-term health of the mother and child (Marshall et al., 2022). Numerous adolescent mothers encounter intricate socioeconomic and lifestyle obstacles that necessitate expert and communal assistance to assist them in optimising their nutrition and other facets of health and social care prior to, during, and subsequent to their pregnancies. Researchers and clinicians of the 20th century held the belief that the fetus, barring acute famine, was "a perfect parasite" (Almond & Currie, 2011) capable of fulfilling its nutritional requirements. During the middle of the 20th century, pregnant women were advised to restrict their mercury consumption and minimize their exposure to the element. Infants diagnosed with LBW were considered thin and relatively unproblematic. Recent research, on the other hand, indicates that a mother's body mass, dietary patterns, and nutritional status have a substantial influence on the fetus health, both prior to and during pregnancy. Overemphasis on weight gain and undernutrition prior to and throughout pregnancy contribute to adverse pregnancy outcomes for both the mother and the fetus. Additionally, complications associated with conception, placenta, embryo, and fetus development, fetal size, and perinatal complications are influenced by these factors (Bodnar et al., 2017).
Conditions during pregnancy
The parents and healthcare providers might be more inclined to synchronise new born nutrition and intake with weight gain goals rather than with maintaining normal fetal in utero growth trajectories. This is especially true if the existing postnatal strategy, which primarily relies on infant weight as the determinant for neonatal intensive care unit or hospital discharge, accelerates catch-up growth for body fat mass excessively quickly. On the other hand, macrosomia or LGA (Large for gestational age) may occur from fetal overnutrition brought on by maternal obesity, diabetes, and excessive fat and sugar intake. These disorders, which give the fetus an excessive amount of glucose and fat, are becoming more prevalent and are linked to several problems. Fasting and pulsatile postprandial hyperglycaemia stimulate the production of fetal insulin, which in turn causes the fetus to store extra glycogen and accumulate fat. This phenomenon is more common in pregnancies complicated by type 1 diabetes (T1DM), GDM, and T2DM, especially when obesity is present (Barbour & Hernandez, 2018). While macrosomia and/or LGA are frequently linked to pregnancies complicated by diabetes mellitus (DM), the majority of these children are delivered to moms who are obese alone, a condition that now affects up to one in three women. When maternal plasma glucose and lipid contents are high, even more fetal fat mass accumulates (Barbour & Hernandez, 2018). According to recent research, fetal fat mass growth in obese pregnancies is primarily driven by maternal triglycerides, which are made available to the fetus by placental lipases that hydrolyse them to free fatty acids (FFAs) and accelerate the fetus accumulation of fat mass (Adank et al., 2020). Although they may retain fat, fetuses have a restricted ability for fatty acid (FA) oxidation. Being overweight during pregnancy may contribute to obesity in later life, but postnatal fat gain, particularly in the first year or two of life, may continue into adulthood and result in childhood obesity (Marshall et al., 2022).
Glucose Metabolism
On the contrary, abnormally high and stable glucose concentrations may impede the fetal capacity to produce insulin and react to glucose stimulation. An increasing body of evidence suggests that sustained elevations in fetal glucose levels can inhibit the development of fetal neurons, leading to a reduction in the number of synapses, dendritic proliferation, and ultimately cognitive function in the progeny. A recent investigation involving adolescent children of women with T1DM in humans unveiled a significant decline in cognitive ability (Bytoft et al., 2016). Children whose mothers had more severe hyperglycemia associated with DM exhibited lower IQ scores and more cognitive difficulties. In addition, major malformations of the central nervous system originating from the neural tube, such as caudal regression syndrome, were more prevalent in the offspring of mothers with both type 1 diabetes mellitus and type 2 diabetes. Moreover, the risk period during organogenesis (less than eight weeks) often occurred before women were aware of their pregnancy. However, near-term stillbirth is most prevalent among mothers with type 2 diabetes, especially when the disease is combined with obesity (Browne, Park, Goetzinger, Caughey, & Yao, 2021; Mackin et al., 2019). Obesity is associated with malnutrition and excessive maternal caloric intake. Moreover, research has demonstrated that maternal overnutrition significantly contributes to the premature initiation of childhood obesity and inflammatory disorders, such as non-alcoholic fatty liver disease (NAFLD), the most prevalent liver disease that impacts one in every three obese children worldwide. According to human data, neonates delivered to mothers who were obese or had gestational diabetes mellitus had 68% more liver fat. This finding is strongly associated with the mother's pre-pregnancy body mass index (BMI) and potentially her triglyceride levels prior to the complete establishment of subcutaneous fat reserves (Hernandez et al., 2019). In addition, even after controlling for childhood BMI, high birthweight (LBW) doubles the risk of advanced fibrosis in children with biopsy-confirmed NAFLD, according to data from the national paediatric non-alcoholic steatohepatitis (NASH) network. For unknown causes, these results suggest that birth abnormalities may foreshadow and/or predict the early onset of NASH in at-risk children. In the absence of appropriate treatment, children with NASH are at risk for developing cirrhosis and dying from liver-related causes in their early adultery (Marshall et al., 2022).
Bone Strength
Pregnancy-related poor eating habits in the mother have been demonstrated to decrease juvenile bone density, increasing the risk of fracture in the children. Our lab has previously established that measurements of long bone development in gestation are correlated with maternal vitamin D levels. Nonetheless, a number of other nutrients—mostly derived from food—may also be important for the formation of an offspring's bones. For the best possible bone health, macro- and micronutrients including calcium, zinc, phosphorus, potassium, and magnesium are all thought to be crucial. Both calcium and phosphorus are essential elements of bone that support bone strength. Furthermore, research has shown that potassium inhibits calcium excretion in the urine, magnesium potentially influences the development of hydroxyapatite crystals, an essential constituent of bone, and zinc potentially functions in enzymes and regulators that aid in the maintenance of the bone matrix. Already established studies have shown that the bone density of children during their childhood is influenced by the amount of minerals consumed by their mothers, such as phosphorus and magnesium. Pregnancy therefore presents a potential important point of intervention for the prevention of osteoporosis in the future; nevertheless, there is currently a deficiency of health promotion that is especially directed towards this demographic. In order to support both the mother's physiological changes and the fetus optimal growth and development throughout pregnancy, a healthy, balanced diet is crucial.
Birth Weight
Poor maternal nutritional status is causally associated with abnormal fetal growth patterns, including macrosomia (>4 - 4.5 kg), large for gestational age (>90% birthweight for gestational age), small for gestational age (<10% birthweight for gestational age), fetal growth restriction (FGR), and low birth weight (LBW; <2500 g). These anomalies are associated with heightened susceptibility to chronic ailments during both childhood and maturity (Marshall et al., 2022).
Determining whether fetal malnutrition in humans arises from maternal malnutrition or other variables including maternal, placental, and/or fetal disease is not always achievable. Birth weight is frequently employed in epidemiological research as a proxy indication of abnormal fetal growth and development because there are no clinical tests that are regularly available to evaluate the optimal fetal growth potential. It was proposed that this link could have its roots in the early life environment, namely in the impact of mothers. Afterwards, it was demonstrated that birth weight and blood pressure, a significant cardiovascular risk factor, had a substantial and inverse connection. The relative risk remained constant throughout birth weight categories, with men from the highest birth weight group having a seven-fold higher chance of having impaired glucose tolerance now than low-birth-weight men. Men who were underweight at birth but later gained weight had the poorest glucose tolerance. It is unknown what molecular processes underlie the link between low birth weight and an increased adult risk of Type 2 diabetes mellitus. Recent research has demonstrated that males with low-birth-weight exhibit decreased expression of GLUT4, protein kinase C, and phosphoinositide 3-kinase p85α and p110β subunits in their muscles. Impaired glucose tolerance does not develop till after these alterations.
When the famine started, babies' birth weight dropped and continued to decline until it reached its lowest point at eighteen weeks. Compared to those exposed early in gestation, individuals exposed to starvation in the last trimester of pregnancy had smaller babies and shorter bodies. At the age of 19, those who experienced hunger during early gestation had higher rates of obesity, whereas those who experienced it during late gestation displayed lower rates of obesity. Early gestational famine exposure was linked to an increased risk of coronary heart disease at 50 years of age. Adults exposed to famine during the middle to late stages of pregnancy showed elevated levels of proinsulin and insulin, as well as impaired glucose tolerance. Moreover, the greatest 2-hour glucose concentrations were seen in those who were born with low birth weights and went on to become obese adults, distinct organ systems therefore have distinct essential time frames. Early maternal nutrition can affect the number and differentiation of cells in the blastocyst, a pre-implantation embryo that is made up of two cell types—the outer cell mass that will form the placenta and the inner cell mass that will become the fetus—playing a crucial role in the physiology of the fetus. Reduced inner cell mass due to maternal nutritional restriction has been linked to reduced birth weight, limited postnatal growth, changed organ/body weight ratios, and the development of hypertension.
Tissue Formation
The most straightforward explanation is that during crucial stages of development, insufficient substrates cause tissues to grow and/or remodel improperly. Inadequate nutrition at these stages may result in permanently lowered cell numbers and/or changed shape when more robust alternative cell types are selected. Fetal tissues grow in a precise sequence and at specific periods. Strong evidence supports this in the kidney; during fetal nephrogenesis, pregnant mice that are denied protein experience a reduction in the number of nephrons in their offspring as well as hypertension in the future. Additionally, research on animals has demonstrated that fetal undernutrition causes tissue remodelling in the pancreas, liver, and brain. Wide-ranging impacts might result from the remodelling of tissues that control metabolic and endocrine processes. For instance, the hypothalamus is the primary brain region that controls feeding behavior and hunger. It also receives and processes information regarding the nutritional status, including the presence of food in the stomach, the flow of fuels, the amount of stored fat, and glycogen. Through projections to other parts of the brain, such as changing eating behavior, distinct populations of hypothalamic cells can either stimulate or decrease the intake of food. Research in animals demonstrates that the dietary environment during fetal development can change the cell proliferation, migration, differentiation, growth, and apoptosis necessary to produce these predictions (Bouret, 2010).
Hormonal Effects
It is believed that the key fetal growth hormones, insulin, and leptin, which react to fetal feeding, control this hypothalamus development. The aforementioned methods include modifications in gene expression, and the impact of fetal nutrition on epigenetic phenomena—like DNA methylation, which plays a part in turning genes on and off—is now of significant interest. DNA methylation patterns are sensitive to the dietary environment and are mostly set during embryogenesis and fetal development. For instance, alterations in the methylation and expression of certain genes related to energy and lipid metabolism appear to be the primary mechanism by which maternal protein restriction during pregnancy results in hypertension and increased obesity in rat offspring (Burdge & Lillycrop, 2010). Folic acid supplementation in the mother's diet prevents the altered methylation and subsequent defects. Childhood obesity has been linked to epigenetic changes in umbilical cord tissue, among other later effects. Because epigenetic patterns are heritable, it may be possible to account for the way even brief changes in fetal feeding may affect body composition and metabolic characteristics across several generations. The remarkable phenomenon in animal models wherein widely different nutritional interventions in the mother (from global nutrient restriction to high-fat feeding) appear to result in the same metabolic syndrome phenotype in the offspring could also be explained by epigenetic perturbations in a small number of critical metabolic genes. Nutritional influences on epigenetic traits may result in the plasticity of early-life phenotype that is central to the notion of fetal programming. Moreover, this kind of plasticity implies that the programming of long-term outcomes might arise from brief and subtle changes in the nutritional environment during developmental stages when nutrient demands for growth are still relatively low, like the periconceptional period and early embryogenesis, rather than from significant nutritional deficits during organogenesis and differentiation. The possibility that epigenetic programming might influence both maternal and paternal nutrition is an additional fascinating feature of this theory. Male mice that were given a low-protein diet from weaning until they reached sexual maturity showed elevated hepatic expression of genes related to the production of lipids and cholesterol (Carone et al., 2010). In the future, one of the intergenerational determinants of health and illness may need to be dads' nutrition just as much as mothers.
The fetus develops chronic FGR (Bergmann, Bergmann, & Dudenhausen, 2008) conditions of severe maternal malnutrition, a rare event of "survival at the expense of growth." Enhanced glucose absorption in peripheral tissues (e.g., skeletal muscle); decreased utilisation of amino acids for protein synthesis and cell proliferation; impaired pancreatic development, growth, and secretion of insulin; emergence of hepatic insulin resistance accompanied by increased glucose production in an ovine model that induced hypoxia and diminished fetal nutrient supply. Presently, it is understood that the FGR phenotype is associated with an elevated risk of obesity, insulin resistance, and diabetes mellitus (DM) in the future, especially when it is followed by excessive caloric consumption in later life. Regretfully, no method has been found that enhances the FGR fetus growth and development after a pregnancy diagnosis. Prior approaches (bed rest, increased diet, maternal oxygen supplementation, and medicine) have either not been successful or have been harmful.
Complications during pregnancy
Nausea and vomiting
Often called morning sickness, nausea and vomiting are common problems during the early stages of pregnancy, although they can happen at any time of the day. The exact cause remains unknown, possibly linked to increased human chorionic gonadotrophin concentration. 90% of affected women experience relief by 16 weeks of gestation, although symptoms usually start between four and seven weeks. Hyperemesis gravidarum (HG) can cause unintended weight loss, electrolyte imbalance, malnutrition due to poor uptake and retention, and dehydration throughout pregnancy in severe and persistent cases. This frequently calls for hospitalization and intensive care. In addition to negatively impacting the physical and mental health of the mother, HG can also affect the growth and health of the offspring. Mismanagement, inconsistent diagnostic criteria, stigma, and insufficient funding have all contributed to difficulties in HG care and research. There is little information on the nutritional status of HG women and how it relates to perinatal outcomes. A comprehensive strategy that incorporates medical interventions, dietary modifications, lifestyle adjustments, supportive care, and patient education is necessary for successful treatment. According to incidence rates, 70% of pregnancies experience nausea, 60% experience vomiting, and 1 in 200 pregnancies result in HG. Hyperemesis has been linked to Helicobacter pylori infection and female fetal sex. Studies have shown that ginger and pyridoxine may reduce symptoms compared to placebo, while the effectiveness of acupuncture, prochlorperazine, promethazine, and metoclopramide remains uncertain. While it is uncommon for nausea and vomiting to cause death during pregnancy, there have been reports of morbidities such as Wernicke's encephalopathy, splenic avulsion, oesophageal rupture, pneumothorax, and acute tubular necrosis.
Hypertension
Preeclampsia, eclampsia, gestational hypertension (after 20 weeks), and chronic hypertension (prior to or during pregnancy) are all classified as hypertensive disorders of pregnancy (College, 2013). Preeclampsia is classified as newly diagnosed hypertension accompanied by severe organ dysfunction, proteinuria, or uteroplacental dysfunction after 20 weeks of gestation (systolic blood pressure of 140 mmHg or diastolic blood pressure of 90 mmHg for a minimum of two occasions, spaced 4 hours apart). Early disruptions in placentation, inflammation, and endothelial damage are all part of the pathogenesis. For example, frequent neurological issues, including visual symptoms, headaches, impaired liver function, renal insufficiency, and thrombocytopenia (Brown, 2018). There have also been reports of uteroplacental dysfunction leading to stillbirth and fetal growth restriction (Rana, Lemoine, Granger, & Karumanchi, 2019). Maternal factors like age, parity, history of preeclampsia, interpregnancy intervals, obesity, ethnicity, and medical conditions increase the risk of preeclampsia. An elevated risk of preeclampsia is linked to inadequate calcium intake. When these disorders manifest in women with chronic hypertension, superimposed preeclampsia on chronic hypertension happens (Poon et al., 2019). Eclampsia is an illness that can be hazardous and arise during or after pregnancy when seizures occur in the setting of preeclampsia. Pregnancy-related hypertensive disorders have been linked to increased rates of illness and death in nations with lower and middle incomes (LMICs) as well as for mothers and newborns (Leal et al., 2020). The majority of pregnancy-related deaths from hypertensive disorders can be avoided with prompt and efficient prenatal care (Gomes et al., 2022).
Gestational Diabetes Mellitus (GDM)
Marked by hyperglycemia and glucose intolerance, abnormalities in the metabolism of proteins, carbs, and fats as a result of reduced insulin secretion, and diminished functional capacity during pregnancy, posing obstetrical and pediatric complications (Hung et al., 2021). In pregnancy, glucose is crucial for fetal development, and the placenta plays a significant part in transporting glucose to the fetus. This transport is mainly facilitated by GLUT1, a type of protein that increases in density as pregnancy progresses to meet the growing fetus needs (Illsley & Baumann, 2020). The capacity for glucose transfer from the placenta to the fetus increases significantly during pregnancy. However, in pregnancies with high blood sugar levels (such as those with GDM), the placenta's development can be affected, leading to various complications such as increased oxidative stress, fetal thrombosis, maternal vascular malperfusion, and an imbalance in vasoactive molecules. These include changes in the placenta's size and weight, as well as alterations in the DNA methylation and gene expression profiles of certain cells in the placenta (Cvitic, B, & K, 2018). Studies have shown contradictory results regarding the expression and activity of GLUT1 in the placenta in pregnancies with GDM, as well as in women with high blood sugar and type 1 diabetes in the first trimester (Parrettini et al., 2020). A retrospective cohort study by Fernández-Alba et al. revealed that malnutrition can have severe effects compared the risk of malnutrition in small for gestational age (SGA) newborns and the risk of overnutrition in large for gestational age (LGA) newborns and customized birth weight references in GDM for fetal macrosomia (Fernández-Alba et al., 2020). Another study showed that almost 16.6% of females were BMI <18.5, i.e., malnourished pre-pregnancy, among all the people considered having been diagnosed with GDM. 6.7% of them were overweight with 25.0 ≤ BMI ≤ 29.9 (Hung et al., 2021).
Recovery period
The postpartum period lasts for 6-8 weeks, which begins following the delivery of the fetus, and persists until uterine involution (Frilasari et al., 2020). Post-delivery, healing and recovery are just as important for the new mother as breastfeeding is for the newborn, as she will have to resume normal life while also catering to its various needs. The postpartum period poses new trials and tribulations such as emotional weakness and loneliness- risen from new responsibilities- leading to depression, extended bleeding in both caesarean-section or episiotomy, and natural deliveries, fatigue, and physical stress. In such time, being nutritionally deficit will only cause more harm and slow down internal healing. A perineal wound occurs between the vulva and the anus, between the two thighs, as a result of an episiotomy or rupture that occurs during childbirth. Whilst the puerperium, this will heal in a week if there is no prevailing infection (Gustirini, Pratama, Maya, & Mardalena, 2019). A high intake of calories, vitamins, fluids, and especially protein, are recommended during the puerperium to attain the right nutritional status, on the other hand, insufficient treatment often results in inflammation or infection. A study in Indonesia by Frilasari et al. revealed that a higher level of nutrition and education or awareness meant quicker healing among a group of antenatal women. Adequate protein intake in postpartum mothers enhances cell regeneration, leading to faster healing of perineal wounds. A well-nourished mother can speed up scar tissue formation and the development of fibrin threads, promoting quicker wound healing and accelerated breast milk production (Frilasari et al., 2020).
For lactating mothers, 1000mg/day of calcium is recommended for the maintenance and production of breast milk. In studies done across populations of various ethnic groups, it was seen that consumption of animal milk and calcium supplements improved bone mineral density which sustained post-delivery, while also keeping pre-eclampsia at bay (Gomes et al., 2022). During pregnancy, the intestinal calcium absorption efficiency doubles, while the mother's skeleton reabsorbs calcium during lactation to supply it for the milk. Women who have high calcium demands during lactation and pregnancy are more likely to experience osteoporosis as a result of bone resorption and calcium loss, which are increased by hormonal changes. These can be detrimental to the physical strength of the woman (Miedany, 2022). Osteopenia is a condition of low bone area and mass, while osteomalacia is related to the deficiency of calcium and vitamin D, and invariably to rickets, seen more times than often in pregnant women (Shlisky et al., 2022). Individuals suffering from osteomalacia may experience fragility fractures, muscle weakness, diffuse bone pain and tenderness (Minisola et al., 2021). Among Japanese school-going children, those born to underweight mothers were found to have abnormal bone mass, which implies a threat of osteoporosis and arthritis later in life (Fujita, Kouda, Ohara, Nakamura, & Iki, 2020). In states of high physiological demands like infancy, pregnancy, lactation, and old age, multiple reasons including malabsorption, dietary restrictions, lactose intolerance, food allergies, or cultural limitations forestall the uptake of the required amount of calcium. As for vitamin D, excessive sunscreen use, high-altitude or indoor living can limit its uptake (Uday & Högler, 2020). Hence, resorting to fortification and supplementation are feasible options, with higher bioavailability of nutrients (Horvath, 2021).
Mothers overall mental health
During a period of four to ten days immediately after childbirth, women may face an episode of depression, which is known as postpartum depression (PPD). When there is an onset of PPD, the mother, child, and family will be impacted. It can affect the pattern of sleep and lactation and can affect parenting (Kroska & N, 2020). Lowered happiness, a noticeable shift in eating and sleep patterns, anxiety, exhaustion, feeling worthless, difficulty focusing, and repeated thoughts of suicide or death are among the symptoms that describe PPD medically. A drop in parenting abilities, characterized by increased aggression, unfavourable interactions, less attentiveness, and hampered communication, might result from failing to treat maternal depression. This might make it more difficult to carry out responsibilities as a parent (Opie, Uldrich, & Ball, 2020).
There hasn’t been much exploration on the correlation of PPD with nutritional deficiency. Recent studies have shown that plasma riboflavin levels and erythrocyte fatty acid composition play a significant role in the development of PPD. Women who consume moderate quantities of vitamin-B2 and n-3 fatty acids have lesser chances of PPD (Lin et al., 2019). It has been observed that socio-economic status of women can influence their chances of developing PPD, as women with low affordability, face more struggles, leading to stress, which can eventually trigger depression. Mothers who do not get access to enough food fail to produce enough breast-milk and this notably bears a huge impact on infants. Reducing the risk and development of PPD may be possible with a balanced maternal diet incorporating fruits, vegetables, fish, grains, legumes, and herbs (Opie et al., 2020).
Maternal morbidity and mortality can be significantly increased by complications during the postpartum period, which expose women to serious risks. In contrast to pregnancy and childbirth, postpartum care receives far less attention. The physical and mental health of mothers after childbirth is very crucial. PPD, anxiety, and traumatic stress disorder, all of which are prevalent at this time, are recognized by women as mental health issues. A major drawback in many cases is that it may be stigmatizing to discuss the intensity and experiences of mental health issues. Even though women who have postpartum depression symptoms frequently have thoughts of suicide, due to various reasons they may strongly oppose discussions about suicidal ideas. Therefore, educating the society about the importance of mental health of mothers soon of childbirth, is essential (Adams et al., 2023). Over the course of time, PPD might increase the likelihood of mental health issues and jeopardize their general wellbeing. Maternal behaviours and the relationship between a mother and her child can be significantly impacted by PPD. PPD-affected mothers may find it difficult to identify and react to their baby's requirements, which can cause delays or irregularities in providing the comfort, feeding, and interaction requirements of their child. The mother and child may become less connected as a result of this diminished responsiveness, which might have an impact on the child's social and emotional growth. PPD-affected mothers may not give their babies as much physical affection, such as hugs, cuddles, and soft strokes. In order to foster a mother's and her child's emotional attachment, a tender touch is essential (Saharoy, Potdukhe, Wanjari, & Taksande, 2023).
Three maternal behavioural lifestyle factors—stress, nutritional deficiency, and physical inactivity—can affect the functioning of the immune system and the central nervous system (CNS) in both the mother and the fetus. As a result, they may raise the risk of neurodevelopmental and mental health disorders. Lowering stress, eating well, and exercising frequently throughout pregnancy and after giving birth may be successful measures to mitigate the detrimental effects of maternal stress and malnutrition/obesity on the growing fetus.
Figure 2
The gut-brain axis of a pregnant female.
Various factors affect the gut-brain axis of the mother and, subsequently, the offspring. They may be 1a. Immune response; 1b. Maternal microbiome and mode of delivery; 1c. Feeding patterns (breastfeeding the infant and maintaining a balanced diet); 1d. Gut microbiota. Nutrition mainly impacts 2a. Neuro-sensory development; 2b. Motor skills; 2c. Cognition; Chronic effects of malnutrition may be 3. Ill mental health; 4. Diseases like Parkinson’s and Alzheimer's

Prevention strategies
Existing research has established a correlation between malnutrition and an increased prevalence of maternal complications, including preeclampsia, gestational diabetes, obesity, and postpartum weight retention. Additionally, complications and adverse effects such as fetal macrosomia, caesarean section, childhood obesity, and adult cardiometabolic disease are also associated with malnutrition (Snetselaar, Jesus, Desilva, & Stoody, 2021). Consequently, continuous nutritional status monitoring should be advocated for in public policy guidelines pertaining to pregnant individuals. This practice generates early warning signs that safeguard both the mother and the unborn child, who warrant comprehensive care from infancy onwards to guarantee nutritional security and enable them to exercise their entitlement to sufficient nourishment. As doing so directly protects the growth, development, and health of the expectant child, assessing a woman's nutritional status during her pregnancy should be one of the most important goals of health services (García et al., 2020). The financial and resource implications are a contributing factor in the unwillingness to give universal nutritional assessments. However, part of the reason for this is that there isn't enough solid proof of its efficacy, or there is uncertainty over whether pregnant women and their carers find the present nutritional evaluation methods to be acceptable. Apart from the well-established significance of adequate nutrition throughout pregnancy, pregnancy is seen as a suitable time to modify the lifestyle of the expectant mother as well as that of her family, leading to better consequences (Anderson et al., 2015). To give moms and their families the best care possible, it is crucial to evaluate the pregnant woman's nutritional state before or early in the pregnancy. Numerous methods are used to conduct nutritional assessments, such as anthropometric measures, food intake assessments, and blood biomarker tests. Every strategy has advantages and disadvantages, and each one offers particular information on certain facets of nutritional status. One can directly evaluate the sufficiency of maternal nutrients and minerals by examining biochemical indicators. While some biomarkers, like plasma hemoglobin levels, are often checked for signs of anaemia, not all nutrients and minerals are routinely tested for these purposes since they are not always practicable or cost-effective in most situations. Although there are differences in the suggested threshold for supplementing, ferritin can be used to test for iron deficiency. Additionally, most studies do not perform the analysis of dietary FA ingestion as indicated by mass spectrometry red blood cell (RBC) FA analyses, which reflect 24 FA species and polyunsaturated essential omega-6 and omega-3 FA intakes. However, the analysis can now be performed on a single blood spot, and the cost has come down to a reasonable amount. It is difficult to evaluate the mothers' food intake; practitioners need to invest a lot of extra time, resources, knowledge, and expertise (Marshall et al., 2022).
However, the most crucial step in preventing diet-related malnutrition is promoting health in women who are pregnant, nursing, and raising infants under the age of two. For the first four to six months of life, human milk is specially designed to satisfy the nutritional demands of healthy babies born on time. It is also linked to a decreased risk of developing chronic illnesses later in life when consumed during infancy. The amount of food a mother consumes during breastfeeding and the amount of nutrients she retains in her adipose tissue both affect the content and production of milk and the nutrients in it. Evidence suggests that exclusive breastfeeding for a minimum of six months protects the mother against type 2 diabetes (T2DM) and lowers the risk of childhood obesity in her kids who have GDM (Marshall et al., 2022). This should be accomplished within the political, social, and cultural context of this community. This public policy measure ought to have been accorded greater prominence in the Sustainable Development Goals guidelines, as opposed to being submerged beneath a global objective that would have given it only a cursory mention alongside other initiatives targeting this demographic. To promote optimal nutrition for a significant proportion of the global populace, existing comprehensive pregnancy care programmes must give precedence to the instruction of expectant families regarding the adoption of culturally suitable healthy lifestyles and dietary practices. Such measures are crucial in averting malnutrition and non-communicable chronic diseases, particularly among pregnant women, breastfeeding mothers, and children below the age of two (Castillo-Matamoros & Poveda, 2021).
As an endeavour to treat the newborn with FGR (Fetal growth retardation) more effectively outside the uterus, current prenatal care for pregnancies with FGR entails fetal observation and delivery of the fetus upon the onset of abnormal physiologic signs. Although a precise dietary regimen for addressing FGR does not exist, it has been demonstrated that uteroplacental gene therapy employing vascular endothelial growth factors can safely accelerate fetal development and reduce the incidence of FGR in sheep (Carr et al., 2014). Furthermore, new research suggests that exercise and dietary assistance before pregnancy may be more effective than throughout pregnancy at promoting a healthy placentation and fetal growth (Potdar, A, & M, 2014).
Pica
Iron deficiency is linked to pica, which is the intake of foods or non-food substances in much higher-than-normal amounts. There is a great deal of disagreement over this connection, particularly whether it is a sign or a cause of anaemia (Cox & Phelan, 2008); and zinc and iron have been mentioned. Cravings for taste, fragrance, or texture are examples of pica, and they can also have a religious connotation. Although geographical and cultural variations exist in terms of the substance utilised and the level of legitimacy of pica, the condition prevalence is sufficient to suggest a physiological basis. 38% of African American prenatal, 20% of women with a familial history of pica, and 31% to 44% of low-income Mexican prenatal. Pica can result in a variety of adverse effects, such as elevated liver tests (in the case of consuming baking powder), hyperkalaemia, gastrointestinal disturbances (e.g., obstruction, perforation), contamination (e.g., lead or parasites), food displacement, excessive weight gain or hyperglycaemia (0.5 cup corn starch contains 14,240 kcals), metabolic alkalosis (one box of baking soda per day) and dental complications. Many women worry that if they give in to hunger, the baby will be lacking something, depending on their cultural beliefs. (Some have mentioned worrying that they may miscarry or that their child won't be happy when they are born). The authors have discovered, nevertheless, that by replacing it with something less hazardous, the demand may be sated. Raw potatoes, jicama, dry oats, baby carrots, and burned toast or tortillas have all been utilized effectively when the mother requires the texture or flavor of dirt. Baking powder sour taste can be replaced with sour candy. Improvement is observed after a few weeks of concomitant treatment for iron deficiency by the authors (Cox & Phelan, 2008).
Conclusion
The greatest threat to the health of humankind and the leading cause of compromised mother and child health is still malnutrition. While pregnancy is among the most physically, emotionally, economically, and nutritionally challenging and demanding phases in a woman’s life, it requires that all the needs are met for not only one but two individuals- the pregnant and the developing fetus. Otherwise, both are at the disposal of varying risks which might be temporary or permanent, as it not only retards growth, development, and healing but also weakens the body making it incapable of combating infections. In this paper, we have aimed at elucidating the various aspects of micro- and macro-nutrient deficit, by exploring their function in maintaining good health and adverse conditions experienced otherwise. Ensuring adequate intake of essential nutrients such as calcium, iron, folic acid, iodine, and vitamin D during pregnancy is critical. This is shown to have a direct impact on the gut, and the subsequent microbiome-brain axis. Strains of Bifidobacterium sp., Lactobacillus sp., and Proteobacterium sp., among others are more prevalent and are boosted in a fiber-rich diet. They also directly influence the hormonal balance in the body, in the absence of which, hormonal intervention is a resort. In most cases, dietary modifications are made and supplements for isolated nutrients, or prescribed rich sources are consumed. Gut microbiota is given so much importance because it also plays a huge role in fetal programming. The "Barker hypothesis, posits that the critical phase of fetal development influences lifelong metabolic and hormonal changes, with maternal factors such as starvation and malnutrition permanently altering organ systems. Malnutrition affects glucose metabolism, bone strength, and tissue formation, while birth weight and hormonal balance are compromised. Nausea is usually a common sighting in the first trimester; however, diet-influenced excessive vomiting is considered a medical condition. Hypertension and high blood sugar are also not worrisome unless above a certain threshold, which has been discussed in detail. Out of the 17 Sustainable Development Goals, the very first 3 goals, namely, no poverty, zero hunger, and good health and well-being, must all be met immediately, as 2.5 million mothers are victims of acute and severe malnutrition. For lactating mothers, a daily intake of 1000mg of calcium is essential for bone health and milk production. Inadequate calcium and vitamin D can lead to conditions like osteoporosis and osteomalacia. Postpartum is accompanied by emotional stress, extended bleeding, and fatigue, which are exacerbated by nutritional deficits. Adequate intake of calories, vitamins, fluids, and protein is essential for wound healing and recovery. This can be tackled by emotional support, exercise, and improved intake, particularly of vitamin B2 and n-3 fatty acids. The review emphasizes the importance of holistic preventative strategies by recognizing societal norms, information gaps, and poverty as major contributors to malnutrition. To improve mother and infant health outcomes, these policies must be global in scope while being locally tailored.
Conflicts of interest
Given his role as Associate Editor, Balamuralikrishnan Balasubramanian has not been involved and has no access to information regarding the peer review of this article. Full responsibility for the editorial process for this article was delegated to Editor-in-Chief Kannan RR Rengasamy. The authors declare that there are no conflicts of interest regarding the publication of this article.
Author contributions
Conceptualization, M.P., B.B.; Writing-original draft preparation, L.P., S.S.H., M.K.D; Selected bibliographic sources, A.C. and A.C; Writing-review & editing, L.P., S.S.H., M.K.D., B.B., M.P., A.C. M.P; and B.B. supervised the overall project, provided critical revisions, and contributed to the final editing of the manuscript All authors have read and agreed to the published version of the manuscript.