INTRODUCTION
Cancer is a major health issue, with Testicular cancer (TC) being the most common type among men aged 14 to 44, following prostate and lung cancers. Its incidence has risen in Western countries over the past twenty years. Both genetic and environmental factors contribute to the development of testicular cancer, with cryptorchidism identified as the most significant risk factor (Cheng et al., 2018). Testicular cancer is usually diagnosed in men aged 20 to 34 and is believed to originate from an early lesion called carcinoma in situ of the testis, also known as “Intratubular Germ Cell Neoplasia (IGCN) or Testicular Intraepithelial Neoplasia (TIN)” (Ziglioli, Maestroni, Dinale, Ciuffreda, & Cortellini, 2011).
In 2020, the “International Agency for Research on Cancer (IARC)” reported 74,458 new cases of testicular cancer all over the world with varying incidence rates from 3 to 12 new cases per 100,000 males per year in Western countries. In Asia and the Middle East, a total of 20,651 cases of testicular cancer were documented. The five countries with the highest numbers of cases were India, China, Japan, Turkey, and Indonesia, reporting 4,638 (22.7%), 4,502 (21.8%), 2,458 (11.9%), 1,605 (7.8%), and 1,497 (7.2%) cases, respectively (Giona, 2022). Radical inguinal orchiectomy is commonly used as a primary treatment for testicular tumors which involves excision of the involved testis along with spermatic cord. After surgery, treatment may involve observation only; retroperitoneal lymph node dissection; radiation therapy, and chemotherapy (Shaw, 2008). Chemotherapy, radiation, and surgery are effective standard treatments but they lead to debilitating side effects impacting quality of life for many patients. Siddha medicine is a traditional system of medicine widely practiced in India especially its southern region, which focused on health promotion and management of various chronic diseases.
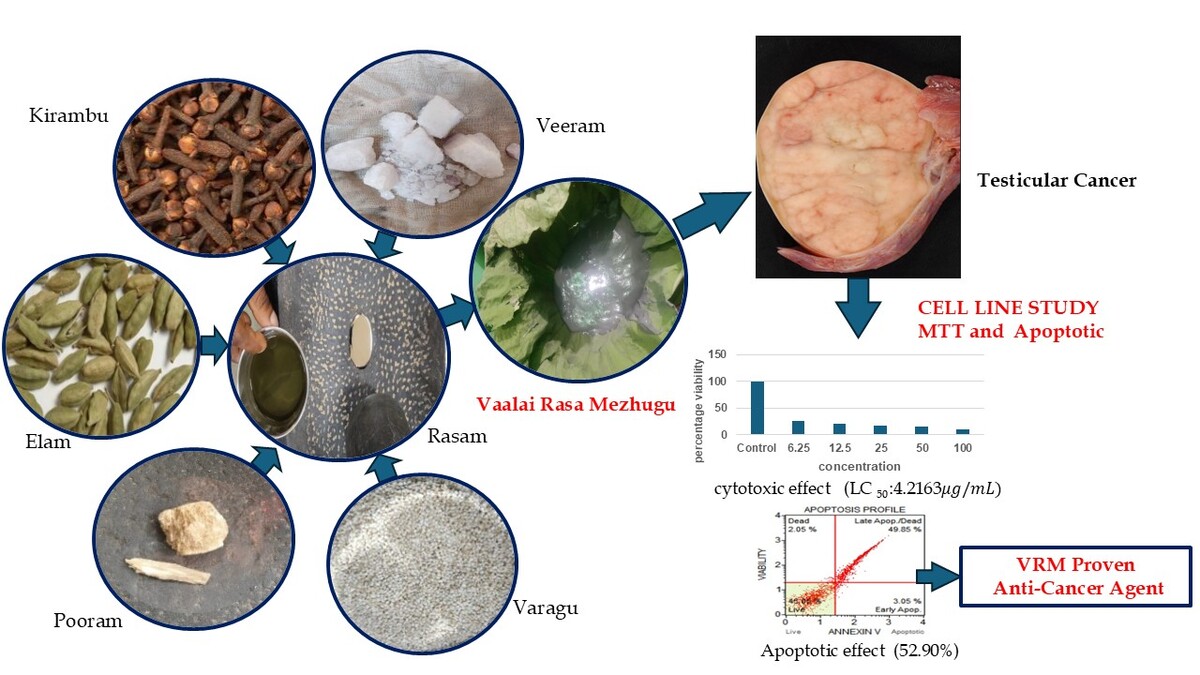
Traditional medicine systems have an important role in fulfilling global healthcare requirements. India has richly diversified traditional healthcare practices namely, Siddha, Ayurveda and others, collectively referred to as Ayush. Siddha system is one of the oldest Indian indigenous traditions having number of formulations with evidence-based anticancer activity for prevention and treatment of various cancers. Vaalai Rasa Mezhugu (VRM) is a Siddha herbo-mineral medicine indicated for conditions such as throbbing pain (Soolai), arthritis (Mudakku vaatham), cervical lymphadenitis (Kandamaalai), fistula (Pauttiram), and lymph node-related neoplasia (Araiyappu). While Siddha physicians have clinically utilized VRM in managing testicular cancer, there is no scientific evidence for its effectiveness (Balarammaiya, 1980; Sambasivampillai, 1991). Rasam (Hydrargyrum), Veeram (Hydrargyrum perchloride), and Pooram (Hydrargyrum subchloride) are the ingredients of major importance in VRM. Numerous studies demonstrated anticancer potentials by these metals. Hence, the present study attempts at evaluating the growth inhibitory effect of Vaalai Rasa Mezhugu (VRM) on the human testicular cancer cell line TM3.
MATERIALS AND METHODS
Selection of the test drug
The test drug “Vaalai Rasa Mezhugu” is one of the Herbo mineral formulations for arthritis and tumor which is indicated in the Siddha literature “Siddha marunthu sei perumuraigal” written by V. Balarammaiya (V. Balarammaiya et al 1980).
Collection of raw drugs
The raw drug was purchased in a Country shop, in Chennai and authenticated by the “Department of Gunapadam, National Institute of Siddha, Chennai” (certified No. NISMB6652024, GUN/AUT/07/24).
Ingredients of Vaalai Rasa Mezhugu
The ingredients of the VRM are listed in Table 1.
Table 1
Ingredients of Vaalai Rasa Mezhugu.
Preparation of Vaalai Rasa Mezhugu
Initially, the Rasam was mixed with betel leaf juice and blended until a smooth consistency was achieved. Next, the Veeram and Pooram were added to the mixture. Then, the clove (Kirambu) and cardamom (Elam) were shallow fried and ground into a fine powder. These powders were combined with Varagarisi flour and blended thoroughly. Finally, a small amount of castor oil was mixed in, and the mixture was ground until it achieved a semisolid, wax-like texture. The final product (VRM) was stored in an airtight container (Balarammaiya, 1980).
In-vitro evaluation of Anticancer Activity
Cell Line
The TM3 cell line (TC) was originally obtained from the National Centre for Cell Sciences (NCCS), Pune, India, and subsequently cultured in Dulbecco's modified Eagle's medium (DMEM).
MTT Assay
Cells were cultured in 25 cm² flasks containing DMEM supplemented with “10% FBS, L-glutamine, sodium bicarbonate, and an antibiotic solution (Merck, Germany)”. The cells were maintained in a humidified 5% CO₂ incubator at 37°C (NBS Eppendorf, Germany). Cell viability was assessed using the MTT assay after direct observation under an inverted phase contrast microscope. Confluent cells were trypsinized, suspended in 10% growth media, and seeded into 96-well plates (5x10³ cells/well). VRM stock solution (1 mg/mL in 0.1% DMSO) was prepared, filtered (0.22 µm Millipore syringe filter), and serially diluted (100, 50, 25, 12.5, 6.25 µg/mL in DMEM). Triplicates of each concentration (100 µL) were added to wells, and incubated at 37°C in a humidified 5% CO₂ incubator. Untreated control cells were maintained in parallel. After 24 hours, cells were examined using an inverted phase contrast microscope (Olympus CKX41 with Optika Pro5 CCD camera), and microscopic observations were photographed. Cytotoxicity markers included cell shape changes, granulation, and vacuolization. 7, 8, 9, 10.
Apoptosis by Annexin V Flow Cytometry
The TM3 cell line was cultured using established methods. Upon reaching confluency, cells were exposed to the LC50 concentration (4.2163 µg/mL) of the sample and incubated for 24 hours. Untreated control wells were maintained in parallel. For apoptosis analysis, cells were transferred to 12 × 75 mm polystyrene tubes (minimum 1 × 10^6 cells per tube). Then centrifugation (5 minutes, 3000 RPM) was done and the resultant supernatant was carefully removed without disturbing the cell pellet. Next, 100 μL of Muse TM Annexin V & Dead Cell Reagent was added to each tube, mixed gently (3-5 seconds), and incubated in the dark at room temperature for 20 minutes. Flow cytometry analysis was performed using Muse software. Cells were gated against untreated controls and evaluated for apoptosis using Muse FCS 3.0 software (Ciniglia, Pinto, Sansone, & Pollio, 2010).
RESULTS
In-vitro Cytotoxic Effect Determination by MTT Assay
Cytotoxic effects of VRM on cancer cells at varying concentrations demonstrating dose-dependent cell viability reduction in Figure.1
Cytotoxicity Assay by MTT Method
Cytotoxic effects of VRM on cancer cells treated with various concentrations of cell viability percentage in Table 2 and Figure 2.
Table 2
Percentage viability of Vaalai Rasa Mezhugu at various concentrations.
Sample Concentration (µg/mL) | Percentage viability |
|---|---|
Control | 100 |
6.25 | 25.88 |
12.5 | 21.82 |
25 | 18.10 |
50 | 15.25 |
100 | 10.97 |
Figure 2
The cytotoxicity of VRM against TM-3 cells was evaluated using the MTTassay, with a graphical representation of results. Cell viability percentages are plotted on the Y-axis, while the X-axis represents varying VRM concentrations. Triplicate experiments yielded mean ± SE values. Statistical analysis employing one-way ANOVA and Dunnett's test revealed significant differences (*p < 0.001) compared to control groups. The LC50 value, signifying 50% cell mortality, was computed using ED50 PLUS V1.0 software, yieldinga value of 4.2163 µg/mL.

Apoptosis by Annexin – V Flow Cytometry
VRM treated induces apoptosis in cancer cells, with notable differences between untreated and treated groups in Figure 3.
The early apoptotic percentage was determined to be 0.10% and the late apoptotic change was found as 4.15%. The total Apoptotic percentage was found to be in the minimal amount of 4.25%.
Figure 3
3(a) Apoptosis profile: untreated controlcells, (b) Cell distribution of cells in control cells.

In the sample treated cells, the early apoptotic percentage was found to be 3.05% and the late apoptotic was found to be 49.85%, 2.05 % constituted the debris resulting in a total apoptosis rate of 52.90%.
DISCUSSION
Standard care for testicular cancer which includes surgical intervention, chemotherapy, and radiotherapy has challenges that cannot be overlooked. Invasive methods may cause infertility or ejaculatory problems (Cheng et al., 2018). Those who get chemotherapy often suffer from nausea, vomiting, and even develop long-term effects (Huddart et al., 2018). Damage to blood vessels an increase in the occurrence of secondary tumors and the heart disease risk are all side effects brought about by radiotherapy (Travis et al., 2010). In addition, each of these interventions might have a deep psychological and emotional impact on the patients aggravating their wellbeing (Smith, Elliott, Regan, Jones, & Lloyd, 2017). Hence it is essential to find new treatments with lesser side effects.
The Siddha system of medicine comprises three fundamental concepts: vali (movement), azhal (conversion), and iyam (form). In essence, azhal is important in cellular digestion and metabolism. It is the overbalance of azhal over iyam (fire over water), which primarily is the feature of tumors, that leads to uncontrolled tissue proliferation. Azhal being low thus creates a state which leads to an excess of the forces of vali and iyam. This may lead to problems like migration owing to the discontinuation of Viyana vaayu, Samana vaayu, and Suzhumunai naadi. Every individual possesses Thegi which is the very unique body constitution of an individual and is a trait dominated by the body time dominated by the particular doshas (Vatham, Pitham, Kapham, and any combinations of these). This constitution also determines how individuals react to the environment, the diseases, and the medications given. To maintain health, an individual must avoid extreme levels of doshas, as is the case with cancer, the most affected doshas being Kapham and Vatham tend to aggravate tumor proliferation and dispersal (Subathra, Gomathi, Shanmugapriya, Ramamurthy, & Meenakumari, 2022).
The MTT assay results indicated that the % of cell viability is inversely proportional to the concentration. At 6.25 µg /mL, it is 25.88, while at 100 µg /mL, it is 10.97. This indicates that cytotoxicity has increased in a dose-dependent approach. It results was 4.2163 µg/mL. This value represents the lethal concentration where 90 % of cells get inhibited upon treatment with the test drug. Annexin V binding assays are used in the detection of apoptosis and prompt discrimination from necrosis, allowing differentiation between late-stage apoptotic and necrotic cells. When annexin V is combined with propidium iodide, it produces an efficacious method to detect and measure apoptosis of testicular cancer cells. The latter was quantified and the fold increase in cell death compared to control represents the apoptotic response of these cells towards treatment with half-maximal lethal concentrations, LC50, (i.e. 90% of cells die at this concentration). Apoptosis was carefully detailed utilising annexin V flow cytometry to provide key information about the cellular response to treatment and, hence, the efficacy of a given therapy. The results indicated that late apoptotic changes in the control group were 4.15%, while the drug-treated group showed a significant increase to 49.85%. Overall, the total apoptotic percentage rose from 4.25% in the control group to 52.90% in the drug-treated group, demonstrating a marked increase in cell death due to the drug's apoptotic activity.
Some of the ingredients in this formulation such as Rasam (Hydrargyrum), Veeram (Hydrargyrum perchloride), and Pooram (Hydrargyrum subchloride) were shown to be cytotoxic and anti-proliferative effects. The anticancer potential of a Siddha preparation of Rathinagarasa Mezhugu (RNM), containing Rasam was analysed using the MCF-7 cancer cell line and the study showed dose-dependent growth inhibition with nuclear damage in cancer cells (Parvathy, Mariappan, & Meenakumari, 2023). 17 in their research on Veera Mezhugu, showed cytotoxicity at 1000 µg/ml against EAC cell lines was 74.05%. These extracts were studied by researchers as Rasa Karpoora kuligai (RSK) with Pooram as the main ingredient to exhibit cytotoxic and apoptotic properties in HeLa cells, thereby reducing cell growth, dose-dependently modulating apoptotic pathways (Manjari, Murugesan, & Saravanababu, 2016). Semalatha S et al. Anti-cancer and anti-tumor effect of Bhramasthiram with Rasam against Human KB oral cell line and HeLa cells was also reported by using MTT assay (Semalatha, 2022). In this study, VRM along with MTT assay results, and Annexin V flow cytometry confirmed that VRM is responsible for its anticancer activity.
CONCLUSION
Vaalai Rasa Mezhugu showed a concentration-dependent decrease in cell viability, indicating substantial cytotoxic activity. The LC50 value confirmed the ability to initiate apoptosis, and evidence from flow cytometry showed a high percentage of late apoptosis in the treated group. However, further in vivo and clinical investigations are needed to prove its therapeutic effect on testicular cancer.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.