Introduction
Lepidium sativum L. (Garden cress) is a medicinal plant and is a member of the family Brassicaceae and edible plant related to mustard. It is native to Egypt and South-West Asia. It is used as vegetables or sources of industrial and cooking oils, forage, and condiments (Alqahtani et al., 2019; Doke & Guha, 2014).Wadhwa, Panwar, Agrawal, Saini, and Patidar (2012) described the plant as an annual, erect herb and can grow up to about 50 cm. The lower leaves are long petiolated and one lyrate Pinnatipartite; the leaves are lanceolate. The inflorescence is in dense racemes. The flowers are 2 mm in diameter and have white or slightly pink petals.
Seed extracts from L. sativum are used as an antirheumatic, diuretic, and febrifuge, as well as for abdominal discomfort, fracture healing, and gout treatment. These seeds have been linked to the treatment and management of a variety of ailments, including asthma, pain, inflammation, nociception, blood coagulation, oxidative stress, anuresis, and other conditions. The presence of several important phytochemical constituents in L. sativum (including alkaloids, flavonoids, cardiotonic glycosides, coumarins, glucosinolates, saponins, sterols, sinapic acid, tannins, triterpene, and uric acid) may be responsible for its ethnopharmacological, preclinical, and clinical recognition (Ghante, Badole, & Bodhankar, 2011).
There are many essential physiological molecules affected by any change in growth conditions. These molecules include photosynthetic pigments, carbohydrates, soluble sugars, proteins and others.Khaleghi, Arzani, Moallemi, and Barzegar (2012) mentioned that chlorophyll is a green molecule in plant cells. It plays a vital role in the photosynthesis process. These molecules absorb sunlight and use their energy to synthesize carbohydrates from carbon dioxide and water. Plants contain two forms of chlorophyll: chlorophyll a and chlorophyll b. Both of them participate in photosynthesis as photoreceptors.
Soluble sugars are categories of carbohydrates. They act as osmolytes to obtain water in the cytoplasm. Similarly, they protect proteins from denaturation and damage to the cell membrane. Furthermore, they induce stability in the structure of enzymatic proteins, thereby preserving their activity (Jafarnia, Akbarinia, Hosseinpour, Sanavi, & Salami, 2018).
Proteins are an essential category of primary biological macromolecules in all living organisms. They are constructed from elements such as oxygen, carbon, sulfur, nitrogen and hydrogen. Proteins are considered as nanoparticles because their size ranged from 1–100 nm. The proteins consist of polymers of several amino acids (polypeptides) consisting of 20 different L-α-amino acids. Proteins have to consists of at least 50 residues to perform all the biological roles, and recently it reached to 300 residues (Ghaly & Alkoaik, 2010).
Some plants genetic diversity has been investigated using different molecular markers (Ahmed, Hosni, Hewidy, Razik, & Bahnasy, 2019). The most effective uses have been the study of molecular variability and phylogenetic relationships, marker-assisted selection, varietal identification, quantitative trait loci (QTLs), or the map-based cloning of genes (Ahmad, Wang, Pan, Sharif, & Gao, 2018). Many identify a limited level of polymorphism despite using different molecular markers to examine genetic diversity in cultivated plant species. Thus, identifying more polymorphic molecular markers is essential for research (Abdein et al., 2018). Other molecular markers estimate the genetic instability and variation in the Populus species' micro-propagated shoots (Paolucci et al., 2010). These molecular markers included AFLP, SSR, RAPD and gene-specific markers.
Lepidium sativum is used as leafy vegetables in salads and appetizers, and it is also used as a medicinal plant as a laxative and aid in improving digestion functions. This work aimed to evaluate the impact of different growth conditions on general plant traits, including the morphology of plants, physiological behavior and also the impact on the genetic stability of the plant.
Materials and Methods
Plant materials
Egypt's Agriculture Center for Genetic Engineering and Biotechnology (ACGEB) gave the seeds of the therapeutic plant Lepidium sativum.
Germination test
Seed germination was performed with three different methods (on different media). (1) In Petri dishes covered with filter paper, incubated at room temperature (25 oC in normal light conditions) (10 seeds/3 dishes). (2) In pots filled with peat-moss soil mixed with sand, incubated at room temperature (25oC in normal light conditions) (10 seeds/3 pots). (3) On Murashige and Skoog (1962) (MS) free medium, incubated at incubator at 25oC with 16h light and 8 h dark (3 seeds/15 jars).
Morphological measurements
After seed germination, five morphological parameters were estimated: “shoot and root length, fresh weight, leaf length and width”.
Physiological bioassay
Three essential physiological components were evaluated for the germinated plantlets: “photosynthetic pigments (chlorophyll A, chlorophyll B and carotenoids), total soluble sugars and total proteins”.
Determination of Photosynthetic Pigments
The quantitative determination of chlorophyll a, chlorophyll b, and carotenoids in the fresh leaves of plants was made using the Metzener, Rau, and Senger (1965) technique. Fresh leaves were powdered in stock acetone to a precise weight (85 percent ). After centrifugation, the supernatant containing the pigments was diluted with 85 percent acetone to a precise volume. The extract was measured at 452, 645, and 664 nm using a colorimeter against a blank of 85 percent aqueous acetone at each wavelength. The following formulae were used to compute the g/ml concentrations of chlorophyll a, b, and carotenoid pigments:
Then, the fractions were calculated as mg/g fresh weight:
Total soluble sugars
Afterwards, a known volume of distilled water was added to the supernatant and a known weight of leaves was ground in 5 ml of ethanol 70%. As described in Umbriet et al. (1959), total sugars were determined using the anthrone technique.: In a 3 ml sample, 6 ml anthrone solution (2 g/l LH2SO4 95 %) was added and kept in a boiling water bath for 3 minutes. It was measured at 620 nm spectrophotometrically after cooling to determine the colour. The concentration is obtained from the following equation:
Concentration (mg/g) =
Total proteins
The total protein content of the leaves was determined using the formula ofBradford (1976). Liquid nitrogen was used to grind the leaves (0.5 g). Each sample was then given 0.5 ml of 2x protein buffer to add to the mixture. After that, the samples were centrifuged at 14000 rpm for 10 minutes at 4 oC, and the supernatants were re-used in fresh tubes. The following formula was used to calculate protein content: Protein reagent (100 mg Coomassie brilliant blue in 50 ml of 95 percent ethanol, then 85 percent phosphoric acid and complete up to 1 litre of water by dH2O) was pipetted into a test tube with 0.1 ml of supernatant. Analyses were carried out using a Spectrophotometer at 595 nanometers. The protein standard curve was used to estimate the concentration of protein. Equation from standard curve calibration was used to compute concentration.:
X (conc.) = (Y (abs.) – 0.030)/0.007 (6)
Molecular marker
DNA isolation and RAPD-PCR bioassay
The CTAB method was used to isolate L. sativum genomic DNA from varied medium growth conditionsEdwards, Johnstone, and Thompson (1991). For 30 minutes at 65 oC, 500 mg of fresh leaves were crushed in 800 μl of 2 percent CTAB buffer and vortexed every 10 minutes. The Eppendorf tubes were centrifuged at 12,000 rpm for 10 minutes before the supernatant was transferred to new tubes. Each tube was filled with equal volumes of chloroform: isoamyl alcohol (24:1) and centrifuged at 12,000 rpm for 10 minutes at 4 oC. Following this procedure, the DNA pellets were separated from water by centrifugation for around two hours at -20oC, and then washed with 70 per cent water to remove any remaining DNA fragments. If necessary, re-suspension can be done with 60 microliters of TE buffer stored at -20 oC.
Eight RAPD primers were used in this bioassay, however only four produced distinct bands, and the primers are listed in Table 2. The Biometra thermocycler was used to execute the RAPD-PCR experiment. Taq master mix (W1020300x, COSMO PCR RED M. Mix, W1020300x), 2 μl of genomic DNA, 1 μl of each primer (Willowfort), and 9.5 μl of water were employed in a total volume of 25 μl for the reaction. 35 cycles of the following processes were performed: 30 seconds of denaturation at 94 oC, 30 seconds of different annealing temperatures for each primer, and a 1-minute extension at 72 oC. This was followed by 10 minutes of final extension at 72 oC, followed by chilling to 4 oC. The amplified PCR product was performed on a 1.2 percent agarose gel in comparison to the (New England Biolab, #N3232S) ladder gel.
Statistical Analysis
Electrophoresis images were examined and the presence of bands was counted as 1, while the absence of bands were counted as 0 in the analysis. These calculations were carried out using Bio-Rad Quantity One (4.6.2) software (Shuaib et al., 2007).
In SPSS 21, the data collected was subjected to variance test analysis. Significant means have been separated using Duncan test in multivariate analysis for all measured parameters (germination percentage, morphology, pigmentation, total soluble sugars and total protein). A dendrogram was carried out using the complete Euclidean linkage cluster in CAP 1.2 software (Community Analysis Package).
Results and Discussion
Morphological and physiological parameters
The L. sativum plant is an essential edible and medicinal plant. Its growth on the different growth media is shown in Figure 1 inside each medium and outside .
The different measured morphological and physiological parameters in the studied plant species were estimated after 2 weeks of germination. All these parameters were illustrated in Table 1.
Morphologically, shoot length has no significant different and all the plants are nearly of the same length. In fresh weight and root length, the plants grown on MS medium are more significant than the others (grown in Petri dish and peat-moss) that are nearly of the same weight. Physiologically, there are significant differences among the plants in different growth conditions in all measured parameters. In the case of seed germination percentage and total soluble sugar concentration, the plant grown on MS medium has the highest value followed by plants grown in peat-moss then Petri-dish. While in case of chlorophyll A, B, carotenoids and total protein concentrations, the plant grown on peat-moss soil has the highest value followed by plants grown in MS medium then Petri-dish. This explained that minerals and different soil constituents enhance the production of pigmentations and proteins. It was observed that the plant tends to lose its chlorophyll pigmentation quickly (after a few days for complete germination). However, the plant grown on MS medium retains chlorophyll content longer than other conditions (on Petri dish and in peat-moss soil).
L. sativum is a medicinal plant with antimicrobial, antioxidant, and anti-inflammatory activities, as mentioned by Alqahtani et al. (2019). Therefore, it is essential to determine the optimum growth conditions to obtain the optimum level for these activities.Lobato et al. (2009) showed that chlorophyll influences total soluble carbohydrates. This enhances indicate that both chlorophyll A and B contents and total soluble sugar concentration increase in case of growth on MS medium. To evaluate any factor’s effect on plant’s morphology, it is essential to estimate many morphological parameters like Roughani, Miri, Hassandokht, Moradi, and Abdossi (2018), who measured twenty-one agro-morphological traits in six accessions of L. sativum to estimate the effect of the geographical distribution on these accessions in Iran.
Table 1
The measured morphological and physiological parameters of Lepidium sativum grown in different growth conditions
Molecular marker (RAPD-PCR
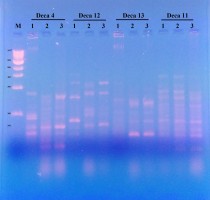
The RAPD-PCR molecular technique had been successfully used in a number of genetic diversity and genetic stability purposes (Figure 2).
Four decamer primers (Deca-4,11, 12 and 13) gave a total number of 39 reproducible bands (as illustrated in Table 2. The total number of polymorphic bands was 19 with a polymorphic percentage of 54.71%. Table 3 indicated the similarity matrix resulted from the four RAPD primers with L. sativum. It indicated that the genetic stability of both seeds germinated in peat-moss soil and on MS media are significantly related to each other, while the genetic stability differs in seeds germinated in Petri-dish.
Different studies used RAPD-PCR molecular technique to distinguish genetic variation or genetic stability of L. sativum. For example,Bansal, Bhasin, Yadav, and Punia (2012) used the RAPD technique to distinguish the genetic variation in 18 genotypes of L. sativum and the mean percentage of polymorphic bands observed was 82.59%. Also,Ottai, Mostafa, and Ibrahim (2012) used the RAPD marker to assess the genetic variation of three cultivars of L. sativum (Haraz, Rajab and Khider) in Egypt. A combination of two molecular markers to assess the genetic variation and genetic stability is available, asKumar et al. (2012) used both ISSR and RAPD markers to estimate genetic diversity among different 19 cultivated genotypes of L. sativum from India.
Table 2
Primer Data analysis of RAPD-PCR bioassay with different media conditions of Lepidium sativum
Table 3
Total similarity matrix resulted from growth in different conditions of Lepidium sativum
| On Petri-dish | On Peat moss | On MS |
On Petri-dish | 100 | 46.18 | 44.18 |
On Peat moss | 46.18 | 100 | 69.9 |
On MS | 44.18 | 69.9 | 100 |
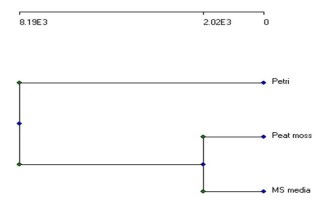
The total relations of the different morphological and physiological parameters and molecular data were gathered in the dendrogram in Figure 3.
This phylogenetic dendrogram is based on complete linkage cluster analysis and illustrates the genetic stability and distance among the different growth conditions of L. sativum. This proved that both plants grown in peat-moss soil and on MS medium are significantly related. At the same time, they are pretty different from one grown in Petri-dish.
Conclusion
This work manipulates the growth of L. sativum seeds in different growth media conditions. These conditions are seeds on petri-dish irrigated with water only, seeds germinated in pots supplemented with peat-moss-sand soil and seed germinated on tissue culture MS media. The measured morphological and physiological parameters varied in response to these different growth conditions. To increase chlorophyll pigmentation, carotenoids and protein compounds it is recommended to use peat-moss-sandy soil; to increase soluble sugars, it is recommended to use MS media culture. The genetic content was affected and varied, resulted in polymorphism percentage of 54.71%. This variation percentage could be led to variation in gene expression. This explained by reflection on morphological and physiological variation.