Introduction
A revolutionary drug delivery technology known as an in-situ gel is a solution that, when exposed to physiological fluids or pH changes, turns into a gel. Drug release can be controlled and sustained due to this special feature. These are the main facts regarding in-situ gels. A formulation that is in solution form prior to entering the body but transforms into a gel form under specific physiological conditions is known as an in-situ gelling system (B. Safirstein, 1976). In-situ gels are easy to administer and reduce the need for frequent dosing, patients find them more convenient and comfortable (Kuhn & Javer, 1998). In-situ gels are used in various drug delivery applications, including ocular drug delivery, oral drug delivery, and more (Kuhn & Javer, 2000). The fungal illness known as allergic fungal rhinosinusitis (AFRS) affects the sinuses and nasal passages. It happens as a result of an allergic response or hypersensitivity to certain fungus in the sinuses. Manning and Holman (1998) they are some Symptoms Nasal congestion and stuffiness, sneezing, nasal polyps, itchy or watery eyes, facial pain or pressure, changes to facial structure (in severe cases) (Ryan & Marple, 2007). A thorough medical history is necessary for certain diagnoses. Nasal endoscopy, which uses a thin tube with a light and camera to visualise the sinuses and identify nasal polyps, is one method used by doctors to confirm the extent of sinus involvement and find fungal masses. Blood or allergy tests are also sometimes used. Find particular allergies caused by fungi (Marple, 2001). Some treatment as an Antifungal medication: These are used to treat AFRS. However, topical antifungals may not be very effective. Corticosteroids: Medications like fluticasone (Flonase) help reduce inflammation and manage symptoms. Biologics: Dupilumab (Dupixent) and omalizumab (Xolair) can reduce inflammation, minimizing the need for steroids and antifungals. Nasal irrigation: Regular sinus flushes with saline solutions improve sinus health by removing mucus and irritants (Bent & Kuhn, 1994). In-situ nasal gel formulations have been extensively studied for enhancing drug delivery efficiency. These formulations can be beneficial for treating allergic rhinitis by providing sustained release and improved bioavailability of herbal drugs like Smilax zeylanica and Tagetes erecta (Manning et al., 1997). The use of mucoadhesive polymers in in-situ gels can prolong drug residence time on nasal mucosa, enhancing drug absorption and therapeutic effects. Additionally, in-situ gels can overcome challenges like rapid mucociliary clearance, ensuring prolonged drug action. By optimizing factors like polymer concentrations and gelation properties, these formulations can be tailored to sustain drug release and improve nasal drug delivery. Therefore, in-situ nasal gel formulations hold promise for effectively delivering herbal drugs to treat allergic rhinitis by enhancing drug bioavailability and providing sustained therapeutic action (Campbell et al., 2006, Ghegan et al., 2006a, Mabry & Mabry, 2000, Mabry et al., 1997, Mabry et al., 1998, McClay et al., 2002, Schubert, 2001, Zinreich et al., 1988). Tagetes erecta Linn (marigold) and Similax zeylanica are two herbal extracts known for their antioxidant and anti-inflammatory properties (B.H. Safirstein, 1976). These extracts have the potential to alleviate allergic rhinitis symptoms by reducing inflammation and modulating immune responses (Ponikau et al., 1999).
Materials and Methodology
The plant materials used in this study were unripe fruits of Tageteserecta Linn (Common name Genda) and leaves of Similax zeylanica (common name kumarika) were collected from the local markets and local area of Dehradun and identified Forest Research Institute Dehradun. Pluronic F127 (Thermo fisher chem.), Carbopol 934 (Lubrizol. chem.), Poly ethylene glycol-4000 (Miralax lab.), Hydroxypropyl Methylcellulose [HPMC K4M] (Alisha lab.), Benzalkonium (Thermo fisher chem.) etc.
Preparation of extract of Tagetes erecta Linn
The gathered plant leaves were cleaned with water and allowed to dry in the shade. A mixer grinder was then used to powder the plant material. The 100g of powder was extracted in a Soxhlet extractor at 40°C using several solvents. Until the solvent in the thimble became clear, the extraction process was continued. This extract was used for additional phytochemical research after being kept in a desiccator. The initial photochemical investigation was carried out (Ferguson et al., 2000, Laury & Wise, 2013, Wise et al., 2008).
Preparation of extract of Similax zeylanica
After washing the leaves (1000g) with distilled water to get rid of dirt and grime, they were well dried in the shade for 5-7 days. After that, they were dried in a tray dryer set at 400°C and ground into powder. They were then extracted using an independent solvent using a Soxhlet apparatus for 72 hours at 55–60°C. The extract was heated to less than 50°C and centrifuged at 85 rpm while being filtered and concentrated under vacuum in a rotary evaporator (Ghegan et al., 2007, Manning et al., 1991, Wise et al., 2004).
Preparation Methods of In-situ gel:
The preparation of in situ gels involves various methods, including:
Cold Method- This method involves slow addition of a polymer solution to a solvent, allowing for the formation of a gel.
Solvent Evaporation Method- This method involves dissolving a polymer in a solvent, followed by evaporation of the solvent to form a gel.
Emulsification Method- This method involves mixing a polymer with a solvent and an emulsifier, allowing for the formation of a gel (Li & Li, 2007, Manning et al., 1993, Schillen et al., 1993).
Preparation of Gel Base:
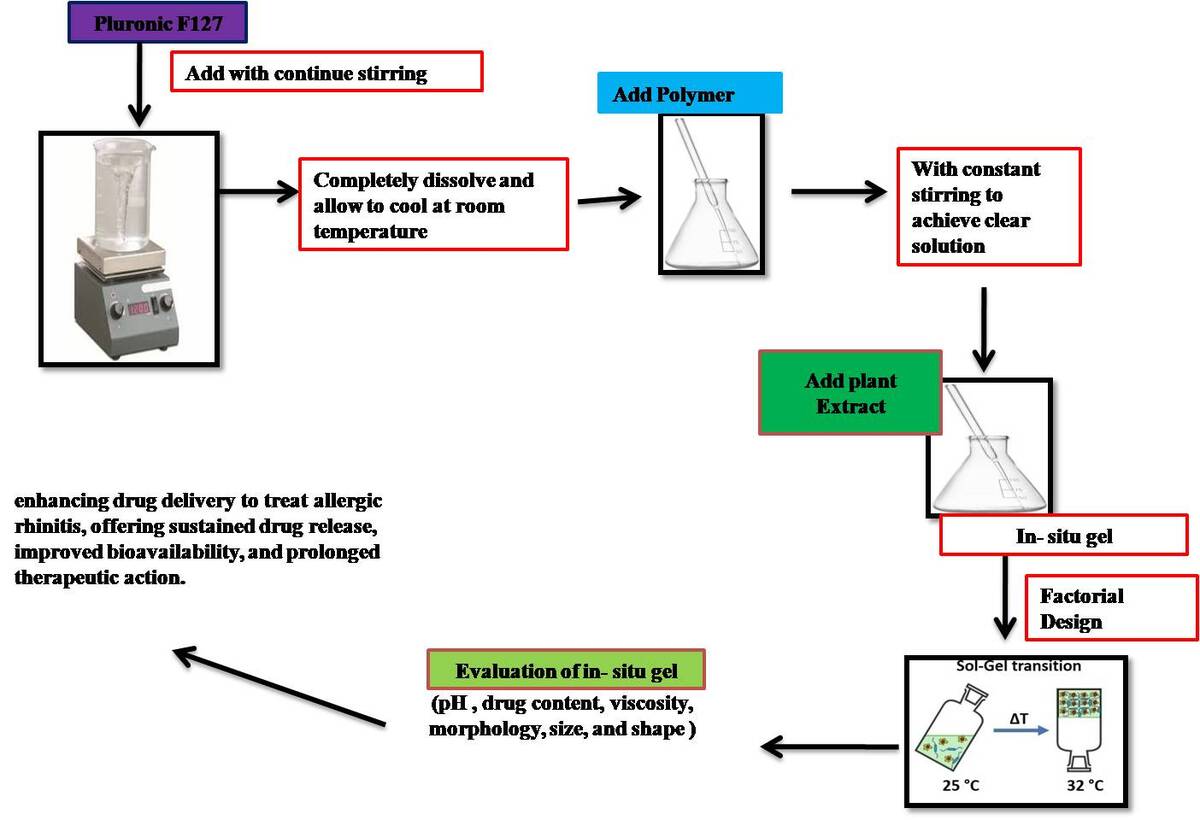
Pluronic F127: Dissolve a predetermined amount of Pluronic F127 in distilled water with constant stirring. Heating may be required to ensure complete dissolution. Allow the solution to cool down to room temperature.
Carbopol 934: Disperse Carbopol 934 in water with constant stirring to avoid lump formation. Allow the dispersion to swell for a few hours, then neutralize with a suitable base (e.g., triethanolamine) to achieve the desired viscosity and pH.
HPMC K4M and PEG 4000: Dissolve HPMC K4M and PEG 4000 in water under constant stirring to achieve a clear solution.
Incorporation of Herbal Extract: Add the standardized herbal extracts of Similax Zeylanica and Tagetes Erecta to the respective polymer solutions/dispersions. Stir gently to ensure uniform dispersion of the herbal extracts in the polymer matrices (Abu-Huwaij et al., 2011, Basu & Bandyopadhyay, 2010, Mainardes et al., 2006, Swathi, 2015).
Using a cold technique, in situ gel was created. Various polymer concentrations were employed, including PEG 4000 (1.5–3% w/v), Carbopol 934 (0.5–1.5 % w/w), and HPMC K4M (0.5–1.5 % w/v) to find the ideal concentration of Pluronic-F127 and other polymers needed for thermotriggered gelling. which, when heated to 32°C, formed thermotriggered gel. By continually stirring on a magnetic stirrer set at 400 rpm, 0.5-1.5% PEG 4000 and 0.5-1.5% Carbopol 934 were dissolved in the aqueous phase to create a thermotriggered in situ gel.
In cold circumstances, 10% Pluronic-F127 was added to the aqueous phase gradually while being constantly stirred. The gel was then filled with this aqueous phase dropwise. To obtain the thermotriggered gel, the solution was kept at 4°C for an entire night following the full solubilisation of Pluronic-F127 (Pratibha et al., 2003). Pluronic-F127 was employed as the thermosensitive agent to produce in situ gel after spraying into the nostrils; carbopol 934 was used as the mucoadhesive agent due to its mucoadhesive capabilities to make the formulation adhere to the mucosal system. Based on the quantity of formulation which the spray pump was capable of to deliver, the active medication had been added.Use of Box Behnken design showing Table 1 and Table 2 shows the composition of the In situ gel (Krishnamoorthy & Mitra, 1998).
Table 1
BBD for optimization range of In-situ gel
Table 2
Use for optimization of Box Behnken design (BBD)
Table 3
Composition of different quantity in situ gel with their responses using BBD
Method of evolution parameter in in-situ gel
Physical appearance: As a visual in gel like colour and transparency.
pH determination: The pH values of the gel samples were measured using a pH meter (Remi Equipment Pvt. Ltd., Kolkata, India). The pH meter was calibrated with buffer solutions with pH values of 4.0, 7.0, and 9.0 before to each use. The pH of the formulation was checked three times, and the mean was calculated.
Drug content: By adding a little quantity of methanol to a 100 mL volumetric flask, which solubilises the gel, one dose (10 mg) equivalent gel formulation was added in order to evaluate the drug concentration. Ultimately, a pH 6.4 phosphate buffer was added to get the volume up to 100 mL. It was thoroughly shaken in a shaker for approximately one hour, then centrifuged and examined. After passing the resulting solution through Whatman filter paper, a UV-visible spectrophotometer was used to determine the absorbance at 228 nm.
Viscosity: The viscosities of the gel were measured using a Brookfield viscometer DVpluse viscometer. equipped with a spindle number DV-II+ PRO. LV1's spindle code is 61. After being immersed in the mixture, the spindle rotated at room temperature for five minutes at a speed of 100 rpm.
In- vitro diffusion Studies: A drug diffusion research conducted in vitro using Hi Media (molecular weight 5000 Daltons) employed a dialysis membrane and a Franz diffusion cell. The membrane used for dialysis was saturated with simulated nasal fluid (SNF) for a whole night. The upper (donor) and lower (receptor) compartments of the Franz diffusion cell were then fitted with the dialysis membrane. The formulation equal to 10 mg of the dose was applied to the dialysis membrane's donor compartment side. The receptor compartment of the Franz diffusion cell was filled with 18 mL of simulated nasal fluid (SNF). Throughout the experiment, the diffusion cells (Remi, India) were kept at 37 ± 0.5°C and stirred at 600 rpm. Using a side tube, 1 mL of aliquots was taken out of the receptor compartment every 15 minutes, 30 minutes, 45 minutes, 60 minutes, 120 minutes, and 180 minutes at predetermined intervals. To keep the sink condition, the same volume of SNF was then added back. The removed samples were examined using 280 nm UV-visible spectrophotometry.
Transmission Electron Microscopy: In order to investigate the morphology of the final gel formulation, transmission electron microscopy (TEM) (H-7500, Hitachi, Kyoto, Japan) was utilised to look at the morphology of the enhanced formulation. The gel was coloured with 1% (w/v) phosphotungstic acid and then placed on copper grids for observation. The shape of the globule was determined by looking at the TEM pictures.
Optimization Statistical Analysis: One-way analysis of variance (ANOVA) was employed in the statistical analysis to compare the outcomes of various formulations. Statistical significance was defined as a p value of 0.05 (GraphPad Prism version 6.03, Boston, MA, USA). The characterisation results were presented as the three experiments' means ± standard deviation (Amkar et al., 2024, Hu et al., 2002, Raut et al., 2023)
Result and Discussion
Physical appearance: As a visual in gel like colour and transparency as result in optimized formulation F-6 is better compare to other formation. (++++) is reparented in excellent property in formulation. its good compatibility and transparency. it showing of observation Table 4.
pH determination: In- situ gel formulation of topical used so skin pH range 5.8 - 7.0 and this formulation best and optimize pH range 6.8 ±0.5 in this formulation batch no. F-6.it is mention of Table 4.
Table 4
Results of gel formulation batch (F1 to F12)
*±S.D (n) =3, * (+) Poor, (++) Good, (+++) Very good, (++++) Excellent
Drug content:The impact of Tagetes erectalinn and similax zeylanica on the drug content in the in situ gel varies. The formulation batch F-6, which includes Carbopol 934 (1.5%), PEG 4000 (2.25%), and HPMC K4M (0.5%), showed the best results for the in-situ gel. The drug content in batch F6 was optimized at 86±0.2%, making it a stable formulation.
In- vitro diffusion Studies:Figure 5 shows how the in-situ gel releases over time after being optimized. The pure drug released quickly, with almost 98% released within 3 hours. On the other hand, the optimized herbal-loaded in-situ gel released about 96.97% after 180 minutes. The optimized formulation (batch F-6) had two phases of release: fast release in the first 180 minutes and then a continuous release afterwards. The herbal gel release was steady over 180 minutes, showing that can trap the herbal drug.
Table 5
Table of In vitro drug release all formulation (F1- F12)
Transmission Electron Microscopy: TEM was used to examine the optimised In-situ gel formulation's size, shape, and structure. As observed in Figure 6, the in-situ gel was condensed into spherical vesicles with distinct walls. The result estimated by TEM was equivalent to the VS obtained using the dynamic light scattering approach. It is representative optimize batch F-6 showing Figure 6.
Optimization statistical analysis: One way ANOVA was applied to the measured values of the examined responses for each of the BBD designs in order to get model equations for the responses that were then analysed using the appropriate statistical tool, Design-Expert 10.0.1 software. Table 6 displays the results of an ANOVA analysis of the chosen replies, which showed that each response surface model was significant and suitable. Based on the two cubic models, the ANOVA results showed that these models were significant for every response parameter. All of the model equation's coefficients are visible. The model equation all response shown in –
Drug content (%) = 0.31+ 0.22 X1 + 1.75 X2 + (0.223x1.758) X1 X2 + (- 0.040) X12 + [(-0.040) x(1.75)]X12 X2 + [(0.223)x(-0.040)] X1 X12 + [(-0.040)x(-0.040)] X12X12
Viscosity (cP)= (-0.25) + 0.31 X1 + 0.12 X2 + (-0.014) X1 X2 + (-0.049) X12 + [(-0.014) x (0.128) X12 X2 + [(0.316) x(-0.049)] X1 X12 + [(-0.049)x(-0.049)] X12X12
Cumulative Drug release at 180 min = 32.2+ 32.8 X1 + 55.7 X2 + (-10.04)X1 X2 + (-5.38) X12 + [(-5.38) x(55.7)]X12 X2 + [(32.8)x(-5.38)] X1 X12 + [(-5.38)x(-5.38)] X12X12
Table 6
Summary of ANOVA for the response parameters
| Response Factor | Sum of square | d.f. | Mean square | F-Value | Prob.>F |
|---|---|---|---|---|---|
| Drug content (%) | 1.25 | 4 | 0.37 | 635.42 | <0.0001 |
| Viscosity (cP) | 0.045 | 4 | 3.25 | 10.56 | 0.0307 |
| Cumulative drug release (CDR 60min.%) | 632.32 | 4 | 145.40 | 41.84 | 0.0036 |
The Contour and 3D response surface graphs were generated by the design expert 10.0.1 software.
Discussions and Conclusion
The study concluded by demonstrating the effective creation and enhancement of in-situ nasal gel formulations for the administration of herbal medications such as Similax zeylanica and Tagetes erecta Linn. Promising outcomes in terms of extended therapeutic effect, enhanced bioavailability, and sustained drug release were demonstrated by the in-situ gels. Drug absorption and therapeutic effects were improved by the formulations' usage of mucoadhesive polymers, which extended the drug's residence duration on the nasal mucosa. The in-situ gels also overcome obstacles such quick mucociliary clearance, guaranteeing sustained drug action. The batch F-6 formulation was shown to be stable and efficacious for nasal drug delivery because to its exceptional physical appearance, pH range, drug content, and viscosity.
The significance and applicability of the developed formulations were validated by means of statistical analysis employing ANOVA and response surface models. The viscosity, cumulative drug release, and drug content model equations showed how independent variables affected the responses, offering insightful information for future research and optimisation. The Design Expert 10.0.1 software produced contour and three-dimensional response surface graphs that demonstrated how the independent variables interacted with one another and affected the responses. Overall, the study highlights how in-situ nasal gel formulations, which provide sustained drug release, enhanced bioavailability, and longer therapeutic activity, could be a promising method for improving the delivery of herbal medications to treat allergic rhinitis.