Introduction
Diabetes, now crossed all boundaries and socioeconomic differences, stood as one of the leading causes of death in the 21st century. With such increased incidence at an alarming rate, diabetes is considered an epidemic. Further, it is estimated that by 2045, 700 million people may be affected by diabetes (Kowluru & Mohammad, 2022). The etiology diverges from the autoimmune destruction of pancreatic β cells followed by insulin deficiency to insulin secretion and insulin resistance acquired through a sedentary lifestyle (Oladoja et al., 2024). Though there are several conventional drugs available in the market to manage diabetes, at least a few drugs can't reverse diabetes to normal and further, each drug is associated with side effects. As these drugs are also used long-term, the cost burden and side effects are added to the patients. This leads to looking after traditional medication by performing broader preclinical and clinical research to combat diabetes and its complications (Oladoja et al., 2024).
In the Unani system of medicine, Diabetes Mellitus is correlated with Dhayābītus based on the resemblance in signs and symptoms. Still, the pathophysiology of both is different. Therefore, the approach and line of treatment are different, although both target decreasing blood glucose levels and minimizing the complications of diabetes. According to the Unani System of Medicine, Dhayābītus is a disease of the kidney that develops due to an increase of abnormal hot temperament (Su"-Mizāj-i-Hārr) in the kidney. Hot temperament disturbs kidney functions and weakens the Quwwat-e-Māsika (retentive faculty) of the kidney, which results in the flow of excessive fluids from the body. The morbid temperament also increases the Quwwat-e-Jādhiba (Absorptive faculty) of the kidney. Quwwat-e-Jādhiba pulls and absorbs excessively more amount of fluid from the liver but is unable to retain and digest the extra fluid due to weak Quwwat-e-Māsika and strong Quwwat-e-Dāfia (expulsive faculty). The fluid ultimately reaches the urinary bladder and excretes in large amounts of urine (Israili, 1907).
Commonly, diabetes is of two types: type I and type II. Streptozotocin (STZ) can be used as an inducing agent to produce both models in rats. STZ is chemically a nitrosourea and antibiotic extracted from Streptomyces achromogenes. STZ selectively destruct the pancreatic β-cells transported via glucose transporter (GLUT-2) present on the plasma membrane of β-cells and induces local immune response followed by cellular toxicity. STZ administration without nicotinamide destroys whole pancreatic β-cells and produces a model for Insulin Dependent Diabetes Mellitus (IDDM) or type I diabetes. While concurrent administration of nicotinamide partially preserves β-cells destruction induced by STZ, and co-administration produces a model for impaired insulin secretion or deficient (Furman, 2015; Masiello et al., 1998).
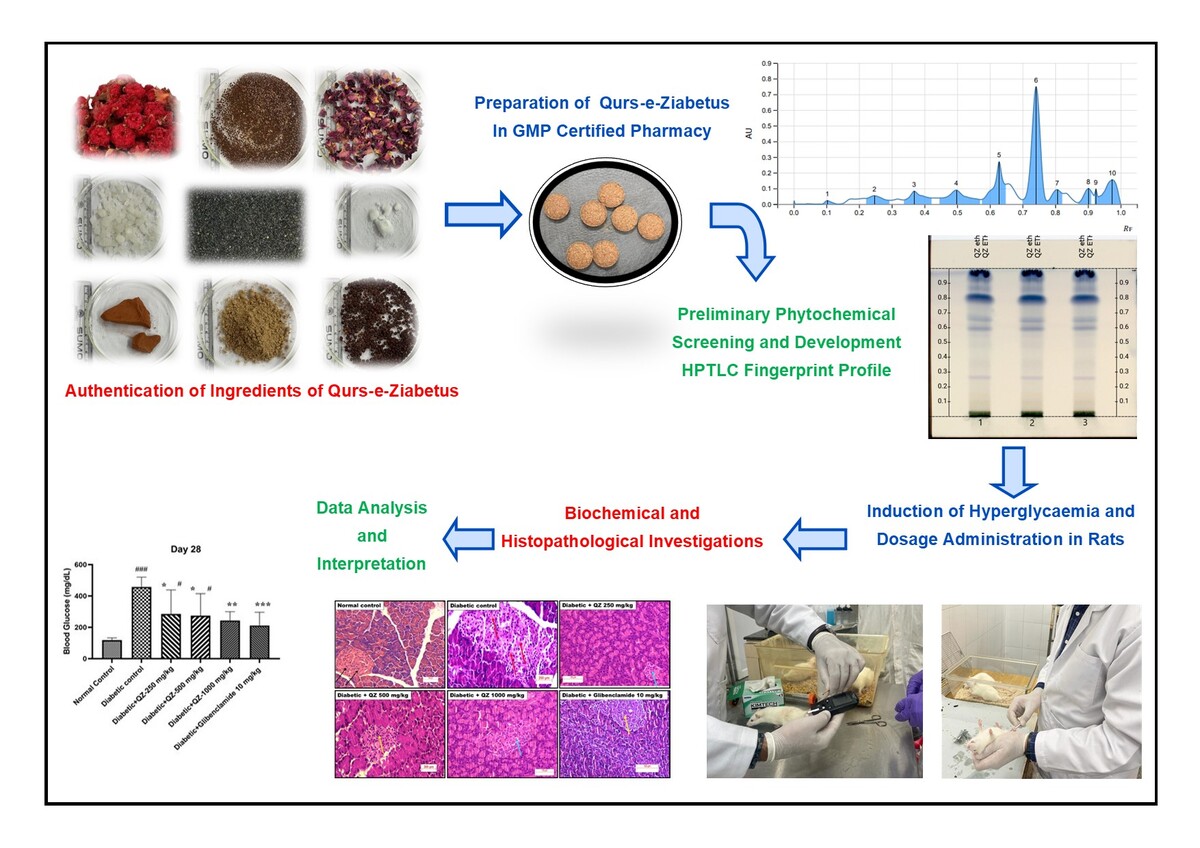
Polyherbal Unani formulation Qurs-e-Ziabetus (QZ) is traditionally indicated for treating diabetes mellitus by Unani physicians (Hafeez, 2005; Kabeeruddin, 2000). This formulation comprises 11 ingredients, as documented in classical Unani literature. We have evaluated the efficacy of QZ in a model of nicotinamide-streptozotocin-induced diabetes to validate the claims of Unani medicine. The present study aimed to assess the antidiabetic activity of QZ in nicotinamide-streptozotocin-induced diabetic rats and to develop an effective, economical, and safe treatment for type II diabetes. Furthermore, this study sought to substantiate the traditional claims regarding the antidiabetic properties of this compound formulation (Hafeez, 2005; Kabeeruddin, 2000).
Materials and Methods
Chemicals and reagents
Streptozotocin (STZ extra pure, 98%, # 14653-1G, CAS: 18883-66-4) was obtained from Sisco Research Laboratories (India) and Glibenclamide (G0382-5G, >98.5% CAS: 10238-21-8) from Tokyo Chemical Industry (Japan). The remainder of the reagents and chemicals acquired from HiMedia Laboratories Private Limited (Thane, Maharashtra, India) were of pure analytical quality.
Formulation/Drug
Herb-mineral Unani drug formulation, Qurs-e-Ziabetus (QZ), composed of eleven ingredients, i.e., Tukhm-e-Khurfa (Portulaca oleracea L.), Tukhm-e-Kahu (Lactuca sativa L.), Tabasheer (Bambusa arundinacea Willd.), Tukhm-e-Hummaz (Rumex vesicarius L.), Gil-e-Armani (Armanian bole), Gulnar Farsi (Punica granatum L.), Gul-e-Surkh (Rosa damascena Mill.), Kafoor (Cinnamomum camphora (L.) Presl), Kishneez khuskh (Coriandrum sativum L.), Burada sandal safaid (Santalum album L.), and Sumaq (Rhus coriaria L.) (Table 1). The medicinal plants were obtained from local suppliers in Hyderabad. The Institute's Botanist Survey of Medicinal Plants validated the identity of the medicinal plants. The voucher specimens were preserved under SMPU/CRI-HYD15165, 15166, 15167, 15168, 15175, 15169, 15171, 15174, 15172 and 15173. QZ formulation was prepared in the GMP-certified pharmacy section of the Institute, according to the composition of the formulation given in the National Formulary of Unani Medicine.
Table 1
Composition of polyherbo-mineral Unani Formulation- Qurs-e-Ziabetus (QZ).
Phytochemical screening
Standard techniques were employed to undertake preliminary phytochemical screening of the QZ Unani formulation for the presence of alkaloids, carbohydrates, glycosides, phenols, resins, saponins, proteins, sterols, terpenes, tannins and flavonoids (Table 2). QZ's HPTLC fingerprint profile was also developed using the method and conditions mentioned in Table 3 (Naikodi, Waheed, Shareef, Ahmad, & Nagaiah, 2011).
Animals
Male Sprague-Dawley rats (8−10 weeks) were obtained from the National Institute of Nutrition, Hyderabad. The animals were acclimated for one week and were maintained under an ambient temperature of 23±2 ℃ and relative humidity of 45-55% with a 12-hour light cycle. Animals had free access to a normal dry pellet diet and water ad libitum. The study was conducted after approval by the Institutional Animal Ethics Committee vide protocol number NRIUMSD/IAEC/17/2022/01/P05.
Table 3
HPTLC instrumental conditions for QZ fingerprint profile.
Dose calculation
As mentioned in Unani literature, the clinical dosage of QZ is 4.5 g daily. Considering the clinical dosage of 4.5 g, the corresponding rat dose is approximately 500 mg/kg (x) based on the body surface area conversion method (Hafeez, 2005; Kabeeruddin, 2000; Reagan-Shaw, Nihal, & Ahmad, 2008). Based on the above dose, it was decided to keep 500 mg/kg as mid-dose. Two more doses, one lower and one higher, were selected as 250 and 1000 mg/kg, respectively, to observe the dose-dependent antidiabetic effect, if any.
Experimental design for evaluation of the effect of QZ on oral glucose tolerance test
The oral glucose tolerance test (OGTT) was performed to estimate the peripheral glucose utilization in euglycemic rats. Rats of either sex were divided into five groups (n=6), fasted overnight, and administered with 0.3% CMC suspension, QZ (250 mg/kg bw, 500 mg/kg bw, 1000 mg/kg bw) and Glibenclamide (10 mg/kg bw), respectively. Glucose (2 gm/kg) was administered orally 30 min after the QZ and glibenclamide treatment. Blood glucose level was determined from blood samples collected from the tail vein at 0 min (before glucose administration), 30, 60 and 120 min after glucose administration as in earlier studies (Bera et al., 2012; Husain, PN, & V, 2009; Jana et al., 2012).
Experimental design for evaluation of the effect of QZ on Nicotinamide-Streptozotocin induced diabetic rats
Induction of nicotinamide-streptozotocin diabetes mellitus: Non-insulin-dependent diabetes mellitus (NIDDM) was induced in overnight fasted male rats by a single i.p. injection of 65 mg/kg streptozotocin, 15 min after a single i.p. administration of 120 mg/kg nicotinamide (Masiello et al., 1998). Current pharmacology protocols were followed to prepare and administer streptozotocin solution (Furman, 2015). The blood was collected from the tail vein after day 7 of streptozotocin injection, and glucose was analyzed using a glucose strip and glucometer (ACCU-CHEK active). Rats with steady hyperglycemia (FBG level more than or equal to 200 mg/dL) were selected for further study. A total of 40 STZ-nicotinamide-treated rats were randomly assigned to groups II – VI (n=8). The day of randomized assignment into different groups was considered day 0 of the experiment, and thereafter, from day 1, the intervention of QZ and Glibenclamide was initiated. Group I was regarded as a normal control and did not receive nicotinamide-STZ (received normal saline i.p.). After induction of hyperglycemia, each group received their assigned treatment for 28 days. Group I and II received 0.3% CMC p.o. as a vehicle, Group III, IV, and V received QZ treatment (250, 500, 1000 mg/kg p.o.), and Group VI received Glibenclamide 10 mg/kg p.o. as standard treatment.
Biochemical and hematological parameters
FBG in all experimental animals was estimated using a glucometer (ACCU-CHEK active) at the end of 2 weeks and 4 weeks of treatment. After 28 days of SZ and Glibenclamide treatment, the overnight fasted rats were sequentially anaesthetized with inhaled isoflurane (EZ Anesthesia 1339) for about 50 - 60 seconds. 2-3 ml of blood was collected using capillary from retro-orbital plexus for biochemical estimation (Fasting glucose, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C), Liver function test and Kidney function test) by using a fully automatic analyzer (Erba EM-200) and hematological investigation using fully automatic hematology analyzer (Nihon Kohden). For biochemical estimation, the blood samples were allowed for complete clotting for about 2-3 hours before centrifuging at 2000 revolutions per minute for 15 minutes. This was aimed at separating the sera from clotted blood cells. The sera were carefully analysed and analysed into new, well-labelled, corresponding plain sample vials at room temperature 23 - 26 ºC.
Statistical analysis
Data presented as a mean± standard error of the mean (SEM). GraphPad was used for statistical analyses. For multiple comparisons, a one‐way analysis of variance (ANOVA) with a post hoc Dunnett’s or Tukey's test was performed. Three levels of significance were reported for p-value (<0.05, <0.01, and <0.001, respectively).
Observations and Results
Phytochemical screening of QZ
Preliminary phytochemical studies of QZ showed the presence of flavonoids, phenols, proteins, glycosides, tannins, terpenes, steroids, saponins, fixed oil, and starch in QZ (Table 2). The fingerprint profile of QZ was developed and presented in Figure 1.
Effect of QZ on Oral glucose tolerance test
An oral glucose tolerance test (OGTT) was conducted in rats to evaluate the effect of vehicle, QZ formulation, and standard Glibenclamide on glucose utilization. There was no significant difference in blood glucose levels among groups at the baseline (before administration of glucose load). In the positive control group treated with glibenclamide 10 mg/kg (p.o.), there was a significant decrease in blood glucose level at 30 minutes (p<0.01), 60 minutes (p<0.001) and 120 minutes (p<0.001) compared to the vehicle control group. No significant difference in blood glucose level was observed in any QZ-treated group compared to the vehicle control group at any time point (Figure 2). This inability of QZ to decrease blood glucose levels revealed that QZ does not directly act on insulin release, as evident in positive control glibenclamide. This could be due to the single-dose administration of QZ (Figure 2).
Effect of QZ on fasting blood glucose in nicotinamide-STZ model
There was a significant rise (p<0.001) in the blood glucose level of all groups on the 7th day of treatment with nicotinamide-STZ compared with normal control animals (administered with vehicle only instead of nicotinamide-STZ) before initiating various interventions (Day-0). After 14 days of treatment with QZ or Glibenclamide, there was a significant increase in blood glucose levels in STZ-treated groups compared with normal control. Glibenclamide or QZ did not significantly attenuate the rise in glucose level compared with the STZ control group. After 28 days of treatment, there was a significant reduction (p<0.001) in blood glucose level in the positive control group treated with Glibenclamide (212±29.90 mg/dL) compared with the nicotinamide-STZ control group (458±23.7 mg/dL). Treatment with QZ for 28 days at 250 mg/kg (286±54.0), 500 mg/kg (274±49.8), and 1000 mg/kg (244±21.3) resulted in a statistically significant reduction (p<0.05, p<0.05, and p<0.01 vs. STZ control, respectively) in blood glucose level (Figure 3).
Figure 3
Blood glucose level after induction of diabetes in STZ-induced model (before starting the drug treatment; Day-0, 14 and 28). #p<0.05and ###p<0.001 vs. Normal Control group, while *p<0.05, **p<0.01 and ***p<0.001vs. Diabetic Control group. Data presented as mean ± SEM; One-way ANOVA followed by Tukey's multiple comparisons test (n=8).

Table 4
Effect of QZ on body weights in diabetic rats.
Effect of QZ on body weights
At day 0 (7th day of nicotinamide-STZ administration), there was a trend of body weight reduction in all nicotinamide-STZ groups (group II to group VI) compared to normal control group. However, no statistically significant difference was observed. On day 7 of the respective treatment, a substantial reduction in body weight was observed in Glibenclamide (p<0.05; 209±6.10g), QZ-500 mg/kg (p<0.05; 214±6.13g) and QZ-1000 mg/kg (p< 0.01; 206±5.47g) compared to normal control (251±9.16g). At Day-14 of treatment, a significant reduction in body weight was observed in diabetic control (p<0.05; 220±5.68g), glibenclamide (p<0.05; 219±5.25g), QZ-250 mg/kg (p<0.05; 225±15.5g), QZ-500 mg/kg (p<0.01; 216±5.81g) and QZ-1000 mg/kg (p< 0.01; 211±4.17g) compared to normal control (263±9.89g). On day 21 of treatment, a significant reduction in body weight was observed in diabetic control (p<0.05; 226±6.47g), glibenclamide (p<0.001; 221±5.84g), QZ-250 mg/kg (p<0.05; 231±16.8g), QZ-500 mg/kg (p<0.01; 220±5.47g) and QZ-1000 mg/kg (p< 0.01; 216±5.16g) compared to normal control (274±10.4g). At Day-28 of treatment, a significant reduction in body weight was observed in diabetic control (p<0.01; 225±6.88g), glibenclamide (p<0.01; 226±4.93g), QZ-250 mg/kg (p<0.01; 230±17.4g), QZ-500 mg/kg (p<0.0001; 212±5.48g) and QZ-1000 mg/kg (p< 0.001; 209±5.63g) compared to normal control (285±10.7g). There were no significant changes in body weights in all the treated groups compared to the diabetic control (nicotinamide-STZ) group (Table 4).
Effect of QZ on Relative Organ Weight
Induction of diabetes resulted in a significant increase in relative organ weight (ROW) of the liver in the diabetic control group (**p<0.01) compared to the normal control group. However, there was a reduction in ROW of the liver in QZ-treated groups, which was not a significant difference compared to the diabetic control group. A significant difference in ROW of the liver was observed in the Glibenclamide 10 mg/kg (#p<0.05) treated group compared to the diabetic control group (Table 5). The ROW of kidneys increased significantly in the diabetic control group and both treated groups (Glibenclamide and QZ) compared to the normal control group. No significant decrease in kidney ROW was observed in the treatment groups (Glibenclamide and QZ) compared to the diabetic control group (Table 5).
Table 5
Effect of QZ on relative organ weight in diabetic rats.
Table 6
Effect of QZ on Liver Function Test in diabetic rats.
Table 7
Effect of QZ on renal function test in diabetic rats:
Table 8
Effect of QZ on serum protein content in diabetic rats:
Table 9
Effect of QZ on lipid profile in diabetic rats.
Effect of QZ on liver function parameters
There was no statistically significant difference in total bilirubin, ALT, and AST levels in any group compared to the normal control group. Though there was up to a 12-fold increase (compared to normal control) in ALP level in a few nicotinamide-STZ-treated rats (including STZ control and another treatment group), the difference was not statistically significant compared to normal control (Table 6).
Effect of QZ on renal function parameters
Uncontrolled diabetes can damage the glomeruli in the kidneys, causing proteinuria (excess protein in urine) and impaired kidney function, often reflected by elevated creatinine and blood urea nitrogen (BUN) levels. High blood glucose can directly damage kidney cells, impacting their ability to filter waste products. There were no statistically significant differences in serum urea, creatinine and uric acid levels between treatment groups compared to normal control group animals (Table 7).
Effect of QZ on serum protein content
There were no significant differences in total protein and albumin levels in any group compared to the normal control (Table 8).
Effect of QZ on lipid profile
There were no significant differences in total cholesterol, triglycerides and HDL levels between the groups compared to normal control, and values remained within normal physiological limits (Table 9).
Effect of QZ on hematological parameters
No statistically significant differences exist in any hematological parameters (except platelet counts) between normal control and other treatment groups. There was a statistically significant decrease in platelet count in the STZ control and glibenclamide-treated groups compared to the normal control group (p<0.05). Statistically significant reduction in platelet count was also observed in QZ-500 (p<0.05) and QZ-1000 (p<0.01) compared to normal control (Table 10).
Effect of QZ on histopathological examination
Hematoxylin and Eosin (H&E)-stained slide of the pancreas revealed that normal beta cells appeared in islets of the pancreas in the normal control group. Degenerative changes in beta cells / acinar cells and islets of the pancreas were observed in all animals in the nicotinamide-STZ control group and further moderate apoptosis/necrosis and haemorrhages. Hypertrophy and hyperplasia of islets of the pancreas with beta cells were observed in 50% in QZ-250, 75% in QZ-500 and 71.4% of QZ-1000 mg/kg group and 62.5% of animals from Glibenclamide. This observation indicated that QZ at 500 1000 mg/kg has a comparable therapeutic effect to standard Glibenclamide (Figure 4). Moderate periductular fibrosis was observed in QZ-250, and in QZ 1000 mg/kg, mild atrophy of islets of the pancreas with apoptosis and degeneration of beta cells was noticed. Still, acinar cells in the pancreas appeared normal (Figure 4).
Table 10
Effect of QZ on hematological parameters in diabetic rats.
Figure 4
Representative photomicrograph showing the effect of QZ and glibenclamide on the pancreas ofnormal rats and of STZ+NAM-induced diabetic rats. The normal control group has normal histology of islets of Langerhans (black arrows). Diabetic control rats showed moderate degeneration of islets (red arrows). Diabetic+QZ 250 and Diabetic +QZ 1000mg/kg showed mild atrophy of islets (blue arrows). Diabetic+ QZ 500mg/kg and Diabetic+ Glibenclamide 10 mg/kg showed mild hypertrophy of islets (yellow arrows).

Normal morphology of glomerulus and tubules of kidneys were observed, and degenerative changes were noticed in 12.5% of animals from the normal control group. In the nicotinamide-STZ control group, severe tubular degeneration cortical tubules epithelial cells, characterized by vacuolation of the cytoplasm and pyknosis of nuclei, was observed and further with a degenerative change noticed in 100% of animals. In the QZ-250 mg/kg group, normal morphology of glomerulus was observed, moderate tubular degeneration cortical tubules epithelial cells characterized by vacuolation of the cytoplasm and pyknosis of nuclei were observed, and degenerative changes noticed in 37.5% of animals. In QZ-500 mg/kg, normal glomerulus morphology was observed, but mild tubular degeneration and haemorrhages were noticed in the cortex region, and further degenerative changes were noticed in 50% of animals. In QZ-1000 mg/kg, moderate tubular degeneration cortical tubules epithelial cells are characterized by vacuolation of the cytoplasm and pyknosis of nuclei was observed. Further degenerative changes were noticed in 71.4% of animals. In the glibenclamide-treated group, moderate tubular degeneration cortical tubule epithelial cells characterized by vacuolation of the cytoplasm and pyknosis of nuclei were observed, and degenerative changes were noticed in 50% of animals. Inflammatory changes in the kidney were not observed in any treatment groups, including the STZ control and glibenclamide-treated group (Figure 5).
Figure 5
Representative photomicrograph showing the effect of QZ and glibenclamide on kidneys of normal rats and of STZ+NAM-induced diabetic rats. Normal control group has normal histology of glomerulus (black arrow) and of tubules (white arrow). In diabetic control group, glomerulus sclerosis (green arrow) and severe tubular degeneration (redarrow) were observed. Diabetic+QZ 250mg/kg showed normal histology of glomerulus (black arrow) and moderate tubular degeneration (yellow arrows) were observed. In Diabetic+ QZ 500mg/kg normal morphology of glomerulus (black arrow) and mild tubular degeneration(blue arrow) were observed. In Diabetic+ QZ 1000 mg/kg and Diabetic+Glibenclamide 10 mg/kg moderate tubular degeneration (yellow arrow) was observed.

Normal morphology of hepatocytes was observed in the portal, peri portal, and centrilobular region of animals in the normal control group. Inflammatory changes were noticed in 57.14% of animals from the nicotinamide-STZ group, and multi-focal necrosis of hepatocytes along with infiltration of lymphocytes and fibrosis were observed in the liver's centrilobular and periportal region. 25% of animals from the glibenclamide group showed inflammatory changes. However, QZ did not show any reactive inflammatory changes in 250, 500 and 1000 mg/kg groups, indicating some protection against STZ-induced toxic effects on the liver in rats, which makes apparent normal morphology of hepatocytes was observed in the portal, periportal and centrilobular region of the liver (Figure 6).
Figure 6
Representative photomicrograph showing the effect of QZ and glibenclamide on livers of normal rats and of STZ+NAM-induced diabetic rats. The normal control group has normal histological architecture, (blackarrow). In diabetic control rats, multi-focal necrosis of hepatocytes along with infiltration of lymphocytes and fibrosis were observed, indicated with red arrow, and steatosis is indicated by blue arrows. All treated groups Diabetic+250,Diabetic+500, Diabetic+1000 mg/kg, and Diabetic+glibenclamide 10 mg/kg showed normal morphology of hepatocytes (black arrows).

Discussion
Preliminary phytochemical studies of QZ showed the sample's presence of flavonoids, phenols, proteins, glycosides, tannins, phytosterols / terpenes, steroids, saponin, fixed oil and starch. Phenolic and flavonoid compounds are reported to improve oxidative stress, lower blood glucose levels, reduce protein glycation, and inhibit other carbohydrate metabolism enzymes, which enhances pancreatic β-cell functions, increasing insulin secretion, reducing insulin resistance and activates many biochemical pathways such as AMPK, PPAR, and NF-κB, which ultimately improve the diabetic condition (Al-Ishaq, Abotaleb, Kubatka, Kajo, & Büsselberg, 2019; Farias, Araujo, Neri-Numa, & Pastore, 2021). A single plant may even contain multiple phytochemical components. In contrast, in a polyherbal formulation, a group of herbs and their several phytochemical constituents exhibit a facilitatory/ potentiation/ synergistic relationship, resulting in beneficial pharmacological action (Suvarna, Shenoy, Hadapad, & Nayak, 2021). The polyherbal approach is more effective and safer when compared to single-plant formulations (Petchi, Vijaya, & Parasuraman, 2014). Polyherbal herbal formulations contain various phytochemicals, which are confirmed in the qualitative estimation of QZ formulation. Any class of phytochemicals has some natural antioxidant activity, which has a combined effect in polyherbal formulations and thereby potentially decreases oxidative stress in diabetic conditions, which is vital for the reversal of diabetes.
Along with their natural tendency to quench free radicals, phytochemicals affect diabetic symptoms and their pathology-related mechanisms. Many flavonoids, isoflavones, which come under the polyphenols class, have been reported to inhibit α-amylase and α-glucosidase from the salivary gland and brush border of the small intestine, thereby inhibiting the rapid breakdown of complex saccharides to glucose. β-glucans are present in some herbal formulations that increase gluconeogenic and glycogen synthesis in diabetic conditions. Phenols and their acid derivatives can increase insulin secretion from β-cells. Alkaloids and heterogeneous polysaccharides present in different polyherbal formulations plantsiminish insulin need by decreasing fasting blood glucose, HbA1c and postprandial blood glucose (Alam et al., 2022).
OGTT test was performed in rats to evaluate the effect of vehicle, QZ and Glibenclamide on glucose sensitivity in rats. Overnight fasted animals were administered with an oral glucose load (2g/kg) 30 minutes after their corresponding treatment. The test determined that glibenclamide-treated rats were observed to have significantly decreased blood glucose levels, estimated at 30-minute, 60-minute, and 120-minute time intervals when compared to the normal control group. In contrast, QZ-treated groups did not decrease blood glucose levels at any time interval compared to the normal control group. This inability of QZ to reduce blood glucose levels revealed that QZ does not directly affect insulin release, like Glibenclamide. This could be due to the single-dose administration of QZ. It has been reported that polyphenol fraction (25 and 50 mg/kg) from Coriandrum sativum treatment for 4 weeks ameliorates alloxan-induced type 1 diabetes in mice by its potent anti-inflammatory and antioxidant activity and further improves liver biomarkers AST and ALT, kidney biomarkers urea and creatinine and serum lipid profile (Mechchate et al., 2021). However, in the same study, 25 and 50 mg/kg doses decreased serum glucose levels in mice even after administering a single dose of the OGTT test. Phenolic acids and flavonoid-rich leaf extract of Bambusa arundinacea at 500 mg/kg show antihyperglycemic activity in both euglycemic and hyperglycemic rats subjected to STZ (Joshi, Patil, Mujawar, Kumar, & Kholkute, 2009).
All the groups of rats administered with Streptozotocin and nicotinamide attained significantly elevated serum glucose levels compared to normal rats. Treatment (Vehicle or Glibenclamide or QZ) was scheduled for 28 days, and the effect of treatment on serum glucose levels was evaluated on days 14 and 28. Till day 14th, there was no significant reduction in serum glucose levels. Continuous treatment to day 28 showed a substantial decrease in serum glucose levels in the treatment groups glibenclamide, QZ-250, 500, and 1000 mg/kg compared to the treated STZ control group. Remarkably, standard treatment glibenclamide and QZ 1000 mg/kg treated animals showed no significant difference in serum glucose levels when compared to normal control animals, which indicates that both treatments attenuated hyperglycemia induced by nicotinamide-STZ to a significant degree. The observed antihyperglycemic activity may be attributable to the reported beneficial effect of QZ ingredients, as reported in various studies.
Phytosterols, fatty acid and amide-rich petroleum ether fraction of Portulaca oleracea extract showed anti-hyperlipidemia activity by improving lipid biochemical parameters in rats subjected to STZ intervention (Nazeam, El-Hefnawy, Omran, & Singab, 2017). Coriandrum sativum extract contains many bioactive compounds like phenolic amide, imidazo pyridazine, and thienopyridines, which further show their antidiabetic activity via inhibiting α-glucosidase enzyme and antioxidant activity by demonstrating ferric reducing antioxidant power (Dhakshayani & Alias, 2022). Apigenin, ellagic acid and gallic acid antioxidants found in Punica granatum Methanolic leaf extract probably show antidiabetic activity in STZ-nicotinamide rat model in decreasing blood glucose levels, glycated hemoglobin, AST, ALT, lipid profile and attenuated pancreas histological changes, via ameliorating lipid peroxidation and further increasing antioxidant status GSH, GPx, SOD and catalase (Pottathil et al., 2020). In their study, (Rahideh et al., 2014) recommend diabetic patients susceptible to cardiovascular complications to take sumac 3 g for months. Santalum album showed a significant decrease in the blood glucose level after two months of administration (Kulkarni, Joglekar, Patil, & Arvindekar, 2012). In a study performed by (Reddy, Ramanjaneyulu, Sabbani, & Choday, 2017), ethanolic extract of leaves of Rumex vesicarius lowered blood glucose levels, possibly due to pancreatic secretion of insulin from β-cells in STZ-induced diabetic rats. Treatment of Hydro-ethanolic seed extract of Rhus coriaria at the doses 200, 300 and 400 mg/kg for 28 consecutive days guards the NIDDM diabetic mice (STZ-nicotinamide model) from being prone to infertility via controlling glucose levels, lipid and liver parameters (Ahangarpour et al., 2017). Polyphenol-rich Lactuca sativa elucidated for chlorogenic acid, cyanidin malonyl-glucoside, and quercetin malonyl-glucoside when orally administered (100 or 300 mg/kg) for eight days acutely reduced hyperglycemia and improved insulin sensitivity in high fat diet-induced obese hyperglycemic mice (Cheng et al., 2014).
(Samarghandian, Borji, & Farkhondeh, 2017) reported that STZ-induced diabetic rats treated with crude polysaccharide extracted from Portulaca oleracea and aqueous seed extract of Portulaca oleracea improved body weights and decreased serum glucose levels, which was achieved by attenuating inflammatory markers TNF- α and IL-6, by alleviating lipid peroxidation marker MDA in liver and improving total oxidant status, further activating repair mechanisms (Samarghandian et al., 2017). In a study conducted in STZ-induced diabetic rats and treated with Qurs Tabasheer, an Unani formulation containing Portulaca oleracea seed, Rosa damascena flower, Punica granatum flower, Bambusa arundinacea dried exudate on node, Lactuca sativa Linn seed, and Armenian bole which are also some of the constituents of QZ formulation attenuated hyperglycemia better than standard treatment glimepiride and improved lipid profile (Siddique, 2023; Wadud, 2022). A carotenoid, Lactucaxanthin extracted from Lactuca sativa, attenuated hyperglycemia significantly in STZ-induced diabetic rats by inhibiting α-glucosidase and amylase enzymes (Gopal et al., 2017).
High blood glucose levels in diabetes can trigger oxidative stress in the liver, causing damage to hepatocytes and potentially leading to elevated liver enzymes like alanine transaminase (ALT) and aspartate transaminase (AST) in the blood. Diabetic conditions can also contribute to fatty liver disease, further impacting liver function, so there was a need to evaluate liver function tests. In nicotinamide-STZ-treated animals, liver function parameters like total bilirubin, ALT, AST, and ALP, though looked elevated to abnormal levels compared to normal control group, were not statistically significant. Animals treated with standard drug glibenclamide and QZ also could not improve liver function parameters when compared to both the vehicle-treated streptozotocin control and normal control groups. Even though our study showed no significant effect of QZ formulation in alleviating liver and renal parameters, some of the plants in this formulation in various studies conducted on STZ-treated diabetic rats revealed improved liver and renal parameters. Lactuca sativa extracted in methanol and its treatment to STZ-induced diabetic rats showed improved blood glucose levels and reports good phenolic, flavonoid content, DPPH antioxidant activity, alleviating lipid peroxidation, inhibiting α-glucosidase, amylase enzymes and promote antioxidant status (SOD, CAT and GSH), finally improved liver and renal functional parameters (Naseem & Ismail, 2022). Cinnamomum camphora and Coriandrum sativum ameliorate inflammation by suppressing TNF-α, IL-6 and MDA levels, which are elevated in STZ-induced diabetes and increased antioxidant status by elevating SOD, CAT and GSH in the liver (Fazmiya et al., 2022). An antioxidant and anti-inflammatory polysaccharide extracted from Portulaca oleracea at 100, 200 and 400 mg/kg bw dose attenuated STZ-induced diabetes in rats by enhancing insulin sensitivity, fasting blood insulin and glucose levels, further decreasing proinflammatory IL-1, TNF-α, MDA and antioxidant SOD in liver tissue (Bai, Zang, Ma, & Xu, 2016). In a study, oral administration of α-santol and sandalwood oil significantly restored and increased liver tissue glycogen levels in diabetic mice. This is due to the insulin-mimetic activity of sandalwood oil extracts in direct peripheral glucose uptake or utilization, and its sesquiterpenoid phytoconstituent might act as an insulin secretagogue on surviving β-cells of the pancreas. Studies showed increased hepatic glutathione S-transferase (GST) activity and sulfhydryl (GSH) superoxide dismutase levels in the hepatic tissue. They reduced MDA and protein carbonylation (Misra & Dey, 2013).
The same trend was observed in the case of renal function parameters (Urea, Creatinine and Uric acid), serum protein content (Total protein and Albumin) and lipid profile (Total Cholesterol, Triglycerides and HDL). Various studies have reported Some plants in formulation to attenuate elevated lipid profiles. Portulaca oleracea aqueous extract with its rich antioxidant constituents like apigenin, gallotannins, quercetin, ascorbic acids, α-tocopherols and trace elements reversed STZ-induced hyperglycemia, cholesterol, triglycerides in rats and improved antioxidant status by augmenting Super Oxide Dismutase (SOD), Catalase (CAT) and Glutathione (GSH) in rats (Mohammed, Kadhim, & Al-Qaisi, 2020). R. damascena flower extract treatment decreases glucose levels and improves lipid profile in insulin-resistant rats by targeting regulatory mechanisms to increase adiponectin and PPARγ protein expression (Mohammadi, Fallah, & Gholamhosseinian, 2017). Coriandrum sativum seeds improved the lipid profile of rats via increasing plasma lecithin cholesterol acyl transferase and β-methyl glutaryl CoA reductase activity when seeds were fed to the rats along with the high-fat diet (Nimish, Sanjay, Nayna, & Jaimik, 2011). Obese–Hyperglycemic–Hyperlipidemic (OHH) Merionesshawi rats treated with C. sativum extract (20 mg/kg) for 30 days decreased serum glucose, insulin resistance and lipid profile like total cholesterol and triglycerides and atherosclerosis indices, demonstrating a cardioprotective activity along with hypoglycemic and hypolipidemic activity (Aissaoui, Zizi, Israili, & Lyoussi, 2011).
In the assessment of hematological parameters, carried out on day 28, only platelets were decreased significantly in vehicle-treated Streptozotocin control, standard drug glibenclamide and QZ 500 and 1000 mg/kg treated groups compared to the normal control group. However, the platelet count in all the groups remained within physiological limits (Delwatta et al., 2018).
Glibenclamide and QZ treatment could not improve the relative organ weight of the kidney compared to the STZ control group. Further, the relative organ weight of kidneys in these groups was significantly augmented, as was the STZ control group, compared to the normal control group. While only standard treatment, Glibenclamide significantly improved the relative organ weight of the liver when compared to the STZ control group. Histopathological findings suggest evidence of beta cell proliferation and regeneration in QZ-treated groups compared to STZ control rats, suggesting a positive correlation with the observed antihyperglycemic effect of QZ in this model. Coriander oil extract, with the presence of abundant polyphenols and linalool component, reversed MDA levels, improved GSH levels and restored pancreatic islets histology by reversing vacuolations in a rat model of dexamethasone-induced insulin resistance (Mahmoud, Ali, Mostafa, Hasan, & Sobeh, 2022) Paarakh, 2017. In STZ-induced diabetic rats, though Punica granatum peel extract treatment didn't significantly decrease glucose levels, liver histology was restored when compared to control with apoptotic hepatocytes and the same with the liver biomarkers AST, ALT and ALP (Faddladdeen & Ojaimi, 2019). In a study, in a rat model of STZ-induced diabetes, ethanolic extract of Rumex vesicarius whole plant at 250 mg/kg reversed hyperglycemia by restoring delayed insulin response, inhibiting glucose absorption in the intestine, and increasing glucose utilization. R. vesicarius treatment also repaired pancreatic tissue and β-cells from vacuolation, granulation and atrophy (Suryavanshi, Gautam, Saxena, Panjwani, & Kumar, 2022). According to (Rahman, Ibrahim, Siddiqui, Ansari, & Ahmad, 2022), the seed extract of Rumex vesicarius contains flavonoids and significantly reduced glucose and lipid levels in the serum of HFD/STZ-induced diabetic rats along with improvement in body weights, liver and kidney parameters. Deteriorated cellular architecture of the liver and kidney due to STZ-induced diabetes was restored in the treatment group. It is, therefore, concluded that the beneficial effect of the ingredients discussed above is at least partly responsible for the observed antihyperglycemic effect of QZ in rats.
Conclusion
The present study revealed that QZ treatment significantly ameliorated the nicotinamide-STZ-induced diabetic condition by decreasing fasting blood glucose level and improving the cellular architecture of the pancreas, kidney, and liver, as observed by histopathological analyses following 28 days of treatment. Therefore, the present study concludes that QZ possesses blood glucose-lowering activity as claimed in classical Unani literature. There is a need for further mechanistic studies at the molecular level to explore the observed antidiabetic activity-related mechanisms of QZ. The present study's limitations include a relatively shorter treatment duration (28 days) and lack of quantification of the active ingredient in the herbo-mineral formulation.
Ethical approval
The present study was approved by the Institutional Animals Ethics Committee (IAEC) vide protocol no. NRIUMSD/IAEC/17/2022/01/P05.
Funding
This study was fully funded by the Central Council for Research in Unani Medicine, Ministry of Ayush, Government of India, New Delhi.
Conflicts of interest
None of the authors disclose any potential conflict of interest related to the present article.
Author contributions
TA: Research concept and design, Collection and/or assembly of data, and Writing the article. EV: Research concept and design, Collection and/or assembly of data and Data analysis and interpretation, D - Writing the article. MARN: Research concept and design, Collection and/or assembly of data, Data analysis and interpretation, Writing the article and Final approval of the article. JIS: Research concept and design and Writing the article.DKD: Research concept and design, Data analysis and interpretation, Writing the article and Critical revision of the article. YIM: D - Writing the article, Critical revision of the article and Final approval of the article.UV: Research concept and design, Collection and/or assembly of data, Writing the article and Final approval of the article. GMH: A - Research concept and design, Collection and/or assembly of data, Data analysis and interpretation, Writing the article, Critical revision of the article and Final approval of the article.