INTRODUCTION
Free radicals play a key role in causing various diseases by inducing oxidative stress and damaging cells. Its functions have been linked to the development of various human conditions. Plant-based antioxidants provide protective benefits by counteracting free radicals, reducing oxidative stress, and enhancing cellular health. These antioxidants are valuable for cancer prevention and are commonly used in medicines, supplements and cosmetics to take advantage of their cytotoxic, antifungal, anti-inflammatory and antibacterial properties.
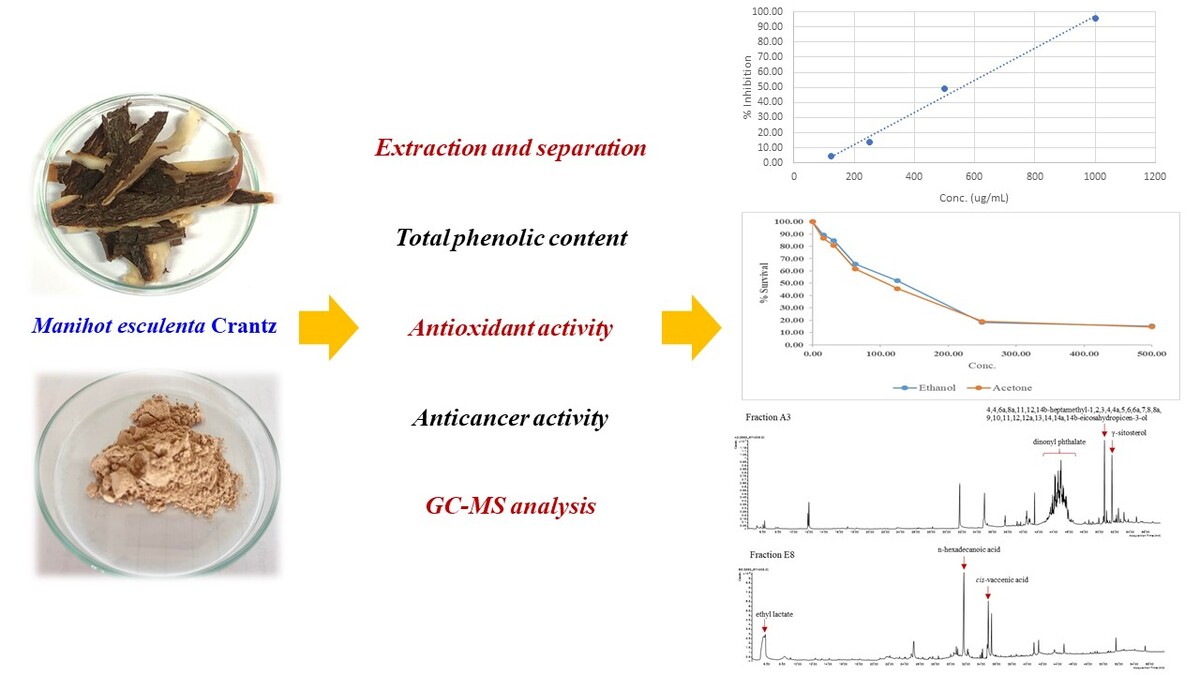
Manihot esculenta Crantz, the cassava, belongs to the Euphorbiaceae family. It is valued for its ability to produce food from various parts of the plant and is primarily cultivated as a root vegetable for humans to eat as a carbohydrate source. It plays a crucial role in traditional medicine, offering a range of health benefits due to its antibacterial, anticancer, antioxidant, anti-diabetic, antidiarrheal, anti-inflammatory, hypocholesterolemic properties (Chinnadurai et al., 2019; Mustarichie, Sulistyaningsih, & Runadi, 2020). Various parts of the cassava plant are used to treat ailments such as diarrhea, wounds, fever, pain, malaria, and blood sugar management. In Malaysia, Indonesia, the Philippines, Latin America, and some other Asian countries, people traditionally eat it as their primary green vegetable (Almazan & Theberge, 1989). Traditionally, the leaves and seeds are utilized for treating a variety of conditions, including fever, headaches, rheumatism, hemorrhoids, pains, snake bites, prostatitis, muscle spasms, and dysentery, as well as for their antiseptic and diuretic properties and in combating cancer-related ailments (Miladiyah, Dayi, & Desrini, 2011; Stintzing, Stintzing, Carle, Frei, & Wrolstad, 2002). It has been found to contain alkaloids, cyanogenic glycosides, and flavonoid glycosides (Osipitan, Sangowusi, Lawal, & Popoola, 2015; Pinto-Zevallos, Pareja, & Ambrogi, 2016). Studies have shown that flavonoids have antioxidant and hypolipidemic properties (Chen & Li, 2007; Otero, Viana, Herrera, & Bonet, 1997) while glycosides are effective in managing heart disease (Feng, Leong, Liu, & Chan, 2016). Furthermore, it is rich in a variety of chemical compounds such as balanophonin, scopoletin, and tannins. They have demonstrated antioxidant, antiproliferative, and anti-inflammatory effects (Yuan et al., 2021).
Despite the traditional and medicinal value of cassava, much of the biomass of the plant, especially the fibrous residues, root peels, and leaves, is considered waste. These residues are commonly used for animal feed and bioenergy, but their chemical composition and potential biological effects have not yet been sufficiently researched. Further research is needed to fully understand the phytochemistry and biological properties of cassava residues. This could open up new avenues for value addition, waste reduction, and sustainability in cassava growing areas. Therefore, the main objective of this work was to determine the phytochemical constituents, free radical scavenging activity using the DPPH method and to investigate the anticancer activity of extracts and fractions from the peels of M. esculenta Crantz. In addition, we aimed to identify the fraction that showed significant activity against DPPH to further analysis of its chemical composition using GC-MS.
MATERIALS AND METHODS
Plant material
The peels of M. esculenta Crantz (Cassava) were collected from April to May 2023 in Tha Kham Subdistrict, Hat Yai District, Songkhla Province. The collected cassava root peels were analysed for foreign matter. Rinse with clean water under running water. Finally, rinse with deionized water. Set aside to drain. Bake in a convection oven at 60oC until the moisture content is less than 5 percent by mass, then grind finely. Store in a vacuum bag and prepare for testing in the next step.
Extraction and separation
A total of 500 grams of powdered coir pith from the peels of M. esculenta Crantz was extracted using acetone at room temperature for a period of 7 days. After the acetone was evaporated, the resulting dark brown gum (6.20 g) was subjected to quantitative column chromatography (QCC) over silica gel, utilizing a gradient solvent system consisting of hexane, 2% acetone/hexane, 50% acetone/hexane, and finally pure methanol, which yielded 6 fractions (A1-A6). Additionally, 500 grams of dried peels from M. esculenta Crantz were extracted in ethanol at room temperature for the same duration of 7 days. The evaporation of the ethanol extract produced a dark brown gum (9.16 g), which was then processed through QCC over silica gel, employing a gradient solvent system of hexane, 5%CH2Cl2/hexane, 70% CH2Cl2/hexane, and finally pure methanol, resulting in 8 fractions (E1-E8).
Determination of Total Phenolic Content (TPC)
The total phenolic content of M. esculenta Crantz peel extracts was determined using Folin-Ciocaltru reagent following modified method of Adusei, Otchere, Oteng, Mensah, and Tei-Mensah (2019). Use gallic acid solution 0.1 mL aliquot of 10, 20, 40, 60, 80 and 100 μg/mL were reference standard for calibration curve. Then mixed 0.1 mL of 10% v/v Folin-Ciocalteu reagent and neutralized with 1 mL of 7.5% w/v Na2CO3 and 4 mL of DI. Allowed to fully react in a dark, room temperature environment for 120 minutes in order to create color. Using a single beam UV-VIS spectrophotometer (BMG LABTECH), the absorbance of the blue color that resulted was measured at 750 nm. Every measurement was done three times for every analysis. The linear equation of a standard curve made using gallic acid was used to calculate the total phenol content, which was then represented as mg of GAE/g of extract. The findings were reported as mean ± SD, and the estimation was carried out in triplicate.
Evaluation of antioxidant activity of crude and fractions
The ability of M. esculenta Crantz peel extracts to scavenge DPPH radicals was investigated using a slightly modified method from a previous study (Rachpirom, Ovatlarnporn, Thengyai, Sontimuang, & Puttarak, 2016). A fresh DPPH working solution was prepared by dissolving DPPH in methanol and the absorbance was measured at 515 nm using a UV spectrophotometer, aiming for an absorbance value of 1.1 ± 0.02. Methanol was used to dilute stock solutions of the herbal extract to obtain a range of concentrations from 100-1000 mg/mL. 70 μl of the sample solution and DPPH solution were combined in a 96-well plate, which was then allowed to stand at room temperature in the dark. Using ascorbic acid as a positive control, the absorbance of the reactions was measured after 30 minutes at 515 nm using a microplate reader. Each experiment was performed three times. The results were expressed as the IC50 value, which indicates the concentration of the sample that results in 50% inhibition. IC50 values were determined by extrapolation from the linear regression equation derived from a graph plotting percent inhibition against sample concentrations.
Cell culture conditions and in vitro cytotoxicity testing
The American Type Culture Collection (ATCC) is the source of human epithelial lung cancer cells (H1792). The Roswell Park Memorial Institute (RPMI-1640) medium, designated as complete media, contains 1% penicillin-streptomycin (50 μg/mL) and 10% fetal bovine serum (FBS). The cell is kept in an incubator with 5% CO2water. Trypsin-EDTA (0.25%) is used to trypsinze cells once they have reached 80–90% confluence. After being sown onto 96-well plates, cells (1.5×104 cells/well) are left for 24 hours. After that, cells are exposed to different concentrations of M. esculenta Crantz extracts for a full 72 hours. Add the MTT, and let it sit for 2 hours. After adding dimethyl sulfoxide (DMSO), incubate for 30 min at room temperature. The absorbance of a colored solution is measured at 570 and 650 nm. To assess a compound's sensitivity, the IC50 is assessed (Maungchanburi et al., 2022).
Gas Chromatography-Mass Spectrometry (GC-MS) analysis
GC–MS analysis of M. esculenta Crantz extracts was performed using an Agilent 7890B GC with an Agilent 5977A Single Quadrupole MS (Agilent Technologies, Santa Clara, CA, USA). A VF-WAXms column (30 m length, 0.25 µm, ID 0.25 mm) was used as the analytical column. The GC was operated in splitless injection mode with the front inlet temperature set to 250°C and an injection volume of 1.0 µL. The oven was set to 50°C for three minutes and then increased to 250°C in twenty minutes at a rate of 5°C per minute. Electron ionisation (EI) at 70 eV was used for MS detection. The interface, MS quad and MS ion source were set to 250°C, 230°C and 150°C respectively. The scan mass range was between 30 and 500 m/z, and the solvent delay time was set to 4 minutes. The components were found by analysing the mass spectra using software from the NIST14 and Wiley 10 database systems. The Office of Scientific Instruments and Testing, PSU, Thailand, was the location where all procedures were performed.
Statistical analysis
Statistical analysis was performed utilizing the Student's t-test and a one-way ANOVA. The analyses and graphical evaluations were carried out using R software version 2.15.1 (http://www.r-project.org/) alongside Microsoft Excel 2007 (Roselle, IL, USA). Results are reported as mean ± SD derived from three distinct observations.
RESULTS AND DISCUSSIONS
This study investigated the phytochemical composition, antioxidant and anticancer activity of the peels of M. esculenta Crantz. The peels were extracted using acetone and ethanol as solvents. The extraction yield from 500 grams of dry peels. Ethanol gave the highest yield with about 1.83%, followed by acetone with 1.24%. The different yields are due to the different compounds extracted by the polar solvent. The efficiency of extraction depends on factors such as the solubility of the compounds. Crude acetone and ethanol were subjected to quick column chromatography (QCC), resulting in the identification of fractions A1-A6 and E1-E8, respectively. The extracts and fractions were analyzed for their total phenolic content and antioxidant properties. In addition, the potential anticancer effect on lung cancer cells (H1792) was investigated for both extracts. From each extract, the fraction with the highest antioxidant activity was selected for further analysis by GC-MS.
Table 1
Total phenolic contentand antioxidant activity (DPPH assay) of M. esculenta Crantz.
Total Phenolic Content (TPC)
Phenolic chemicals are a widely used class of secondary metabolites in plant biology that are important for a number of different processes. These include protection against oxidative stress, defense against infection, possible anticancer properties, and preventive mechanisms against these diseases (Rahman et al., 2021). We performed the tests listed in Table 1 to determine the total phenolic content of the extracts and fractions of M. esculenta Crantz. The TPC was determined in different extracts or fractions (A1-A6, E1-E8). The acetone extract exhibited a TPC of 222.12 mg GAE/g, while the ethanol extract showed a TPC of 178.79 mg GAE/g. Fractions (A1-A6) obtained from crude acetone had a TPC values of 172.00±0.45, 167.18±0.66, 216.09±0.68, 334.71±0.55, 395.77±0.65, and 177.60±0.61 mg GAE/g, respectively. Among these fractions, A5 displayed the highest TPC. Similarly, fractions (E1-E8) from crude ethanol were found to have TPC values of 188.61±0.57, 104.48±0.52, 160.86±0.42, 331.18±0.44, 617.21±0.62, 222.85±0.54, 166.57±0.79, and 81.45±0.48 mg GAE/g, respectively, with E5 exhibiting the highest TPC.
The acetone extract, with a TPC ranging from 167.18 to 395.77 mg GAE/g, exhibits a higher average TPC than the ethanol extract, which ranges from 81.45 to 617.21 mg GAE/g. This indicates that acetone may be more efficient in extracting phenolic compounds. However, ethanol demonstrates the potential for extremely high phenolic content in specific fraction E5, though with greater variability. The differences in phenolic content across various fractions of M. esculenta Crantz are affected by several factors, including solvent selectivity, extraction efficiency, the specific composition of phenolic compounds, the fractionation process, and the inherent biological variability of the plant material. This aligns with findings from previous studies (Bitwell, Indra, Luke, & Kakoma, 2023; Nawaz, Shad, Rehman, Andaleeb, & Ullah, 2020). According to Linn and Myint (2018), the ethanol and aqueous extracts from the leaves of M. esculenta Crantz contained TPC of 748.22 μg GAE/mg and 446.22 μg GAE/mg, respectively (Linn & Myint, 2018). Additionally, the TPC from the leaves using hexane, chloroform and ethyl acetate were 5.35 ± 0.66 mg GAE/g, 7.52 ± 0.09 mg GAE/g and 13.47 ± 0.56 mg GAE/g, respectively (Khan, Yusufzai, Kimin, & Jabi, 2018). The peels of the cassava were extracted with 80% methanol showing the highest phenolic content with 681.5 mg GAE/g (Ekeledo, Latif, Abass, & Müller, 2020). Our investigation demonstrated that the TPC in the ethanol extract was measured at 178.79 mg GAE/g, which is lower than the previously documented TPC of 748.22 mg GAE/g in ethanol leaves extract. In a similar vein, the TPC in the previously reported aqueous extract (446.22 mg GAE/g) exceeded that of any of the fractions we examined. This consistency in results indicates that diverse solvents and extraction techniques can produce varying TPC values. Our findings also indicated that extracts from hexane, chloroform, and ethyl acetate exhibited lower TPC levels compared to acetone and ethanol extracts and their respective fractions. This variability can be ascribed to factors such as the specific plant components utilized, extraction methods, solvent choices, plant species, growth conditions, soil composition, climate, environmental variables, postharvest treatment, processing methodologies, and storage conditions, as highlighted in earlier research byNaczk and Shahidi (2006) and Dusuki, Bakar, Bakar, Ismail, and Azman (2020). The substantial phenolic content in peels extracted with 80% methanol, as documented by Ekeledo et al. (2020), further emphasizes the influence of plant components and solvent selections on TPC.
Antioxidant activity
Antioxidant activity is the ability of compounds to counteract free radicals or inhibit oxidative damage in biological organisms. Antioxidants are compounds that can provide an electron to free radicals without becoming unstable themselves, effectively eliminating the threat. In this study, we investigated the inhibition of free radicals by extracts of M. esculenta Crantz using the DPPH method. The extracts were prepared at concentrations in the range of 100-1000 mg/mL to evaluate their antioxidant activity. The samples were then measured at of 515 nm using a spectrophotometer and compared with standard ascorbic acid solutions. The results showed that the solution turned yellow upon reaction with the DPPH radical, indicating the presence of antioxidant activity.
The antioxidant properties of acetone and ethanol extracts, as well as various fractions, were assessed using the DPPH assay (Table 1). Results indicated that the ethanol extract had a more potent effect compared to the acetone extract, with IC50 values of 575.14 ± 0.24 µg/mL and 621.55 ± 0.15 µg/mL, respectively. The fractions A3, A4, A5, E3, E5, E7, and E8 displayed notable antioxidant activity with IC50 values of 385.83 ± 0.12, 507.87 ± 0.01, 504.69 ± 0.04, 537.56 ± 0.30, 534.04 ± 0.56, 552.78 ± 0.58 and 508.26 ± 0.33 µg/mL, respectively (IC50 value of ascorbic acid 0.5550±1.35 µg/mL). Conversely, lower levels of antioxidant activity were observed in the acetone extract, ethanolic extract, and fractions A1, A2, A6, E1, E2, E4, and E6, with IC50 values ranging from 565.53 ± 0.26 µg/mL to 894.30 ± 0.14 µg/mL. This indicates that the fractions with higher antioxidant activity may contain compounds that contribute significantly to the overall antioxidant potential. According to the results, several isolated fractions of a particular substance exhibit higher levels of DPPH activity compared to crude extracts. Crude extracts may contain non-antioxidant components that may interfere with the measurement of antioxidant activity. Fractionation can lead to the isolation and concentration of specific bioactive compounds, such as phenolic compounds, flavonoids or other antioxidants, which may be present in lower amounts in the crude extract. Fractionation can result in fractions with higher purity, reducing the presence of inactive or less active compounds that may be present in the crude extract. It's important to note that the effectiveness of fractionation depends on the particular plant material, the extraction method and the properties of the target compounds. Researchers often use different techniques, such as chromatography, to fractionate plant extracts and isolate specific fractions for further analysis.
A previous study examining the antioxidants extracted from various parts of this plant found that the ethyl acetate and n-butanol fractions showed considerable scavenging activity against DPPH• and ABTS·+ (Yi et al., 2011). The ethanol extract of cassava leaves had a higher free radical scavenging activity with an IC50 value of 17.69 μg/mL, while the aqueous extract had a lower value with an IC50 value of 42.64 μg/mL (Linn & Myint, 2018). As well as, in a study conducted by Khan and colleagues in 2018, it was found that the ethyl acetate extract exhibited greater antioxidant activity, with an IC50 value of 0.19 mg/mL, compared to the chloroform (IC50 = 1.39 mg/mL) and hexane extracts (IC50 = 1.74 mg/mL). These values were also compared to the standard antioxidant BHT, which had an IC50 value of 0.04 mg/mL (Khan et al., 2018). Comparing our findings with previous research reveals some fascinating insights. While our acetone and ethanol extracts show moderate antioxidant activity compared to the results reported byLinn and Myint (2018) and Khan et al. (2018), specific fractions (A3 and E8) display significantly higher activity. This suggests that certain components within these fractions might be more influential in enhancing antioxidant effectiveness.
Anticancer screening
Cancer is a serious disease that severely affects people. Plants have bioactive compounds that can be used as drugs to treat cancer and to develop new anticancer drugs. The aim of this study was to discover potential new anticancer drugs from natural sources. We have investigated for potential sources of selective anticancer drugs in from acetone and ethanol extracts on lung cancer cells (H1792) (Table 2 ).
Table 2
Screening for selective cytotoxic activity acetone and ethanol extracts on lung cancer cells (H1792).
Sample | IC50 (µg/mL) | Classifies cytotoxic |
|---|---|---|
|
|
|
Acetone extract | 115.80±2.57 | moderated cytotoxicity |
EtOH extract | 111.33±2.25 | moderated cytotoxicity |
Doxorubicin | 0.9735±0.01 | - |
The acetone extract showed moderate cytotoxicity on lung cancer cells (H1792) with an IC50 value of 115.80 ± 2.57 µg/mL, while the ethanol extract had a slightly lower IC50 of 111.33 ± 2.25 µg/mL. These values indicate that both extracts possess comparable effectiveness in extracting bioactive compounds that exhibit cytotoxic effects on lung cancer cells, as classified by the United States National Cancer Institute. These results indicate that the ethanol extract is marginally more effective in reducing cell viability compared to the acetone extract. The observed disparity between antioxidant activity and cytotoxicity may be attributed to the presence of different bioactive compounds in each extract. Although the ethanol extract exhibited lower total phenolic content, its higher antioxidant activity and comparable cytotoxicity suggest that it contains more potent compounds with significant anticancer properties. Conversely, while the acetone extract has a higher phenolic content, its lower antioxidant activity and similar cytotoxic effects imply that the phenolic compounds alone do not fully explain the cytotoxicity. These findings underscore the complexity of the relationship between phenolic content, antioxidant activity, and cytotoxicity. The results suggest that other compounds within the extracts may contribute to their biological activities.
In the extraction of cassava with acetone and ethanol, many active ingredients are obtained in crude extracts, such as polyphenols, glycosides, saponins, and flavonoids, which are a group of substances with antioxidant, anti-inflammatory and anticancer properties. However, in this study, only phenolic compounds, a major prevalent in various plant extracts, have been extensively studied for their anticancer effects, particularly against lung cancer. (Song et al., 2021; Tanagornmeatar, Chaotham, Sritularak, Likhitwitayawuid, & Chanvorachote, 2014). The number and arrangement of hydroxyl groups in polyphenols are crucial for determining their biological activities, particularly their antioxidant and antitumor properties. A higher number of hydroxyl groups enhances the compound's ability to donate hydrogen atoms, which strengthens its antioxidant capacity by neutralizing free radicals and reducing oxidative stress, a factor in cancer development. Additionally, the positioning of these hydroxyl groups affects how polyphenols interact with cellular targets like enzymes, receptors, and membranes, influencing their ability to inhibit cancer cell growth. Certain configurations also enable polyphenols to chelate metal ions, preventing the formation of harmful reactive oxygen species. Therefore, both the quantity and arrangement of hydroxyl groups play a key role in modulating the effectiveness of polyphenols in combating cancer and oxidative damage (Zhou, Pan, & Li, 2019). However, phenolic compounds can interfere with the accuracy of MTT assays, which are used to measure cell viability by detecting the reduction of MTT to formazan by cellular enzymes. Due to their own reducing properties, phenolic compounds can chemically reduce MTT to formazan even in the absence of living cells, creating false positive results. This interference can lead to an overestimation of cell viability or an underestimation of cytotoxicity, making it important to use alternative methods or controls when evaluating the effects of phenolic compounds in such assays. Polyphenols like (-)-epigallocatechin-3-gallate (EGCG) from green tea can interfere with the MTT assay, leading to inaccurate results. This interference occurs because EGCG can chemically reduce MTT to formazan without involving living cells, causing an overestimation of cell viability and an underestimation of its true antiproliferative or cytotoxic effects. Studies byUlukaya, Colakogullari, and Wood (2004) andWang, Henning, and Heber (2010) demonstrated that this issue can result in the MTT test failing to accurately measure the full extent of EGCG's ability to inhibit cancer cell growth, thereby masking its actual anticancer potential. The underestimation of (-)-epigallocatechin-3-gallate (EGCG) cytotoxicity in MTT assays is primarily due to two factors. First, EGCG enhances the activity of mitochondrial dehydrogenase enzymes, which increases the reduction of MTT to formazan, giving a misleading indication of cell viability and metabolic activity. Second, EGCG possesses the inherent ability to reduce both MTT and MTS directly, independent of cellular processes. This direct reducing property further inflates formazan production, masking the true extent of EGCG's antiproliferative effects. Together, these mechanisms contribute to the inaccurate assessment of EGCG's effectiveness in inhibiting cancer cell growth when evaluated using the MTT assay (Ulukaya et al., 2004; Wang et al., 2010). In addition, research by Somayaji and Shastry (2021) reveals a critical limitation of MTT assays when assessing cell viability in the presence of flavonoids like quercetin, (-)-epigallocatechin-3-gallate (EGCG), rutin, and resveratrol. These compounds can chemically reduce MTT to formazan even in the absence of living cells, acting as reducing agents that donate electrons to the MTT molecule. This reduction leads to increased formazan production, creating a misleading impression of cell viability and metabolic activity. Consequently, the presence of these flavonoids can skew results, suggesting that more cells are viable than actually are. This highlights the need for caution and the use of alternative methods or controls in MTT assays to accurately evaluate the effects of flavonoids on cell health (Somayaji & Shastry, 2021). In our study, the moderate cytotoxicity found in the acetone and ethanol extracts might reflect this non-specific MTT reduction. Nonetheless, the observed IC50 values align with those reported for other plant-derived extracts in cancer cell studies, suggesting that the anticancer activity is likely not solely attributable to this interference.
Table 3
GC-MS analysis of fractions A3 and E8.
GC-MS analysis
The extracts and fraction were assessed for their ability to scavenge DPPH. The results indicate that the highest level of antioxidant activity was found in fractions A3 and E8 from crude acetone and crude ethanol, respectively. As a result, two fractions were continuously examined using gas chromatography–mass spectrometry (GC–MS). The GC–MS chromatogram of the identified compounds is shown in Figure 1. The identified chemical constituents are listed in Table 3 by retention time, peak area (%), molecular formula, match factor (>80.00) and % of Total (>1). The GC–MS chromatograms of the A3 and E8 fractions show a total of 6 and 11 components, respectively. From the GC–MS results, the identical two chemicals were present in both fractions, including n-hexadecanoic acid and γ-sitosterol. Analysis of GC-MS data from fraction A3 revealed that 1,2-benzenedicarboxylic acid dinonyl ester was the predominant compound, comprising 50.59% of the total peak area. This was followed by 4,4,6a,8a,11,12,14b-heptamethyl-1,2,3,4,4a,5,6,6a,7,8,8a,9,10,11,12,12a,13,14,
14a,14b-eico-sahydropicen-3-ol (4.74%), g-sitosterol (4.62%), n-hexadecanoic acid (4.03%), oleic acid (4.03%), and lithium bis(trifluoromethanesulfonyl)imide (1.5%). Conversely, analysis of fraction E8 showed a different composition with n-hexadecanoic acid accounted for 17.45 % of the total peak area, followed by ethyl lactate (12.79%), cis-vaccenic acid (9.69%), 1-heptadecanecarboxylic acid (5.89 %), D-glucose (5.62%), and γ-sitosterol (1.64%). From GC-MS data, these results indicate that the chemical composition of isolated fractions varied based on solvent polarity, containing a diverse array of compounds such as fatty acids, sugars, fatty acids esters, glycerol derivatives, phthalates, amides and terpenoids.
The leaves extract of M. esculenta was examined using GC-MS analysis, revealing a diverse range of chemical compounds depending on the solvent used.Khan et al. (2018) identified several compounds in different solvent extracts. In the hexane extract, notable compounds included hexadecanoic acid methyl ester, phytol, 9-octadecenoic acid methyl ester, and squalene. The chloroform extract contained β-amyrin, phytol, 1,4-methanoazulen-9-ol, humulane-1,6-dien-3-ol, and others. The ethyl acetate extract was found to contain squalene, 9,11-dimethyltetracyclo[7.3.1.0(2.7).1(7.11)]tetradecane, and humulane-1,6-dien-3-ol.Manjula et al. (2020) reported additional compounds, including 9,12-octadecadienoic acid, alpha-tocopherol, lupeol, and hexanedioic acid bis(2-ethylhexyl) ester.Mohan et al. (2023) identified new compounds in the methanol extract, such as 1,7-dimethyl-4-(1-methylethyl)cyclodecane, pentanoic acid, 5-hydroxy-2,4-di-t-butylphenyl esters, dibutyl phthalate, and bis(2-ethylhexyl) phthalate. Most recently,Rabiu, Yusha’u, Abdullahi, and Muhammed (2024) highlighted the methanol extract’s significant components, including 9,12-octadecadienal, hexadecanoic acid, 9-oxabicyclo[6.1.0]nonane, and oleic acid. The compounds identified in our study exhibit both similarities and differences when compared to previously reported data. For instance, n-hexadecanoic acid and oleic acid are consistently reported across multiple studies, including those byKhan et al. (2018) and Rabiu et al. (2024), as well as in our analysis. Moreover, the identification of γ-sitosterol in both fraction A3 and E8 aligns with its previously reported presence in the chloroform extract (Khan et al., 2018). The chemical profiles identified in fractions A3 and E8 are distinct from those reported in the literature, highlighting the influence of solvent polarity, the specific part of the plant analyzed, and the methods of extraction employed. The presence of phthalates in both our study and previous research raises questions about their origin, whether as natural products or contaminants. The diversity of compounds, including fatty acids, terpenoids, and phthalates, suggests the complex chemical nature of M. esculenta and underscores the importance of extraction techniques in determining the composition of plant extracts.
The detection of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) in fraction A3 of M. esculenta raises considerable concerns about its origin. LiTFSI, a synthetic compound commonly used as an electrolyte in lithium-ion batteries, does not occur naturally in plant systems. Therefore, the presence of LiTFSI in M. esculenta likely indicates contamination during one or more stages of the experimental process, including sample collection, preparation, or analysis. Possible sources of contamination could be the use of laboratory equipment, reagents, or solvents that contain or have come into contact with LiTFSI. In addition, the limitations of GC-MS analysis, particularly the risk of spectral overlap or interference from other compounds, could lead to misidentification or a false positive result. The detection of phthalates in M. esculenta Crantz (cassava) raises important concerns, as these compounds are traditionally regarded as environmental contaminants. However, phthalates have also been reported in various natural environments, including in plants. The primary phthalates identified in cassava include dinonyl phthalate and bis(2-ethylhexyl) phthalate (DEHP). There is ongoing debate regarding whether these phthalates are biosynthesized by the plant or if their presence is due to external contamination. Given that phthalates are widely used in plastic production and are known to leach into the environment, their occurrence in cassava may be attributed to environmental contamination, potentially linked to agricultural practices, packaging, or processing materials. Furthermore, phthalates found in cassava and other plants could result from the uptake of contaminated soil, water, or air, particularly in regions with significant plastic pollution. As such, further research is needed to determine whether M. esculenta synthesizes these compounds naturally or accumulates them from external sources. Notably,Wahengbam et al. (2023) reported the presence of phthalic acid, specifically di(2-propylpentyl) ester, as one of the major compounds identified in cassava tubers during postharvest physiological deterioration. This compound accumulates as waste within raw cassava root tissues, and its distribution is influenced by its movement through apoplastic and symplastic pathways within the roots (Penido et al., 2019). While phthalic acid is a plasticizer known for its toxicity, its concentration decreases when cassava tubers are boiled before consumption. In addition, dinonyl phthalate, or 1,2-benzenedicarboxylic acid dinonyl ester, was found to comprise approximately 24.19% of the total compounds identified in the leaves of Viola caspia subsp. sylvestrioides (Gharari, Sharafi, Bagheri, Yazdinezhad, & Bijani, 2019). This compound has also been purified and isolated from Rosa laevigata, where it demonstrated protective effects against amyloid-β-peptide-induced neurotoxicity (Choi et al., 2009). Moreover, other phthalates, such as dibutyl phthalate and DEHP, have been identified in the leaves of R. laevigata (Freire, Ramos, & Schwan, 2015; Mohan et al., 2023). Phthalates have been detected in a wide range of organisms, including plants, algae, bacteria, and fungi, suggesting their ubiquitous presence in the environment. For instance, diethyl phthalate has been found in the skin of water caltrop and the roots of Echinochloa crus-galli (Liang & Peng, 2006; Xuan, Chung, Khanh, & Tawata, 2006), while DEHP has been detected in the leaves, roots, and bark of Alchornea cordifolia (Mavar-Manga et al., 2008). Similarly, dioctyl phthalate and butyl isobutyl phthalate have been identified in the petroleum ether and ethyl acetate fractions of Dracaena cochinensis (Wei, Wen, Liu, & Tang, 1998). In addition, dioctyl terephthalate and DEHP were extracted from Capparis spinosa leaves (Nageswara et al., 2000), while bis(2-methylheptyl) phthalate was found in the aerial parts of Hypericum hyssopifolium (Cakir et al., 2003). Di-isooctyl phthalate was also detected in Limonium bicolor (Wei & Wang, 2006), and DEHP was identified in the roots of Euphorbia hylonoma (Ruan, Zhang, Zhang, Pi, & Wu, 2006). Moreover, the antibacterial activity of dibutyl phthalate has been reported in Begonia malabarica (Shobi & Viswanathan, 2018), further emphasizes their complex biological roles, though their potential toxicity in plants and humans continues to raise concerns. Ultimately, more research is required to conclusively determine whether M. esculenta biosynthesizes phthalates or if they are absorbed from external sources, which may have significant implications for food safety and environmental health.
CONCLUSION
The study demonstrates that various fractions of peel extracts from M. esculenta Crantz possess notable antioxidant properties and are rich in phenolic compounds. Additionally, the crude extracts showed moderate activity against lung cancer cells (H1792), indicating promising applications for cassava waste in fields such as pharmacology, medicine, and cosmetics, among others. This plant has been reported to contain 1,2-benzenedicarboxylic acid dinonyl ester and bis(2-ethylhexyl) phthalate for the first time. Future research should focus on isolating and identifying the specific phenolic compounds that contribute to these antioxidant and anticancer effects. Moreover, additional studies should investigate other biological properties, including antimicrobial and anti-inflammatory activities. It is essential to develop practical applications and products that utilize the bioactive compounds present in cassava peels.
Funding
This work was supported by Rajamangala University of Technology Srivijaya, Thailand through the Basic Research Fund for the fiscal year 2023.
Author contributions
Conceptualization: Phetkul U, Chirandorn T; Methodology and Investigation: Phetkul U, Chirandorn T, Khongthong S, Roekngam N, Maungchanburi S, Chaichan K; Supervision and Validation: Phetkul U, Chirandorn T Data analysis and interpretation: Phetkul U, Chirandorn T, Khongthong S, Roekngam N, Maungchanburi S; Writing the article: Phetkul U, Chirandorn T, Khongthong S, Roekngam N, Maungchanburi S; Critical revision of the article: Phetkul U; Final Approval and Submission: Phetkul U.