The supplementary information associated with this article available at https://doi.org/10.53365/nrfhh/200473
Introduction
With the onset of human civilizations plants have been explored as a remedy to treat acute to chronic diseases (Raskin et al., 2002). They have been considered one of nature's most indispensable gifts to humans to sustain their well-being. The current scenario of using plants or herbs in treating diseases is quite astounding (Strange & Scott, 2005). The data revealed that for the forecast period 2022-2029, the compound annual growth rate of the herbal drug market will increase by 11.16% (Bhattacharjee et al., 2024). One of the major concerns or challenges in the herbal drug industry is to ensure its quality and efficacy. It is generalized that different factors viz. temperature, rainfall, humidity, soil, pest, transportation, storage, etc. will influence the quality of plant raw materials. So, it becomes extremely important to adhere to standards framed by WHO and other Pharmacopoeias while dealing with herbal drugs. Strictly adopting standardization parameters will improve the quality of plant-based drugs. Proper authentication and identification of medicinal plants via Pharmacognostic parameters will ensure the quality and purity of drugs (Yadav et al., 2011). Furthermore, the hybridization of wild varieties, advanced cultivation practices, and chromatographic fingerprinting will robust quality control parameters of herbal drugs (Werner, 2000; Stilo et al., 2021). Genus Buddleja (Scrophulariaceae) comprises more than 150 species among which several species have been used ethnomedicinally too (Hikal et al., 2022). The fragrant nectar-containing flowers of Buddleja serve as a potential food source for several insects, butterflies, moths, etc. (Corbet, 2000). Plants belonging to the Buddleja genus are also likely to flourish under calcium-rich alkaline conditions (Green, 2013) e.g., Buddleja utahensis (Meyer, 1976). Various other species have been adapted well to such conditions as B. davidii, B. madagascariensis, B. marrubiifolia (Powell, 1997). Calcicoles have the unique ability to tolerate high calcium concentrations. Some of the members of Brassicaceae are calciotrophic which can accumulate a large amount of soluble calcium in vacuoles thereby providing a decisive role in osmoregulation, particularly in dry limestone areas (Kinzel et al., 1992). Ectomycorrhizal fungus aids in promoting tolerance in plants inhabited in calcareous soil conditions (Lapeyrie et al., 1990). The fungus can release siderophores, produce organic acids, and enhance Fe acquisition which aids in dissolving calcium phosphates thereby promulgating adaptive behaviours in plants (Bolan et al., 1991). It is evident from the literature that mycorrhizal fungal associations existed in two Buddleja species viz. B. davidii (Dickie et al., 2007) and B. cordobensis (Fracchia et al., 2009) but fewer studies have been conducted on B. crispa concerning its root fungal associations. Buddlejas is also the storehouse of iridoids along with other secondary metabolites viz. flavonoids, steroids, and aromatic esters (Khan et al., 2019). B. crispa Benth., also referred to as Himalayan Butterfly Bush, is a deciduous erect shrub that thrives primarily over 1400 meters on exposed rocky cliffs. It is spread throughout the Himalayas, spanning from India, and China to Pakistan. This deciduous shrub has opposite branches that have oblong or ovate leaves on woolly petioles, and it is about 2-3 meters tall and rarely found as a tree around 5-6 meters in height (Liao et al., 2015; Wu & Raven, 1996). Several complex secondary metabolites have also been isolated from B. crispa viz. aryl esters, ginipin (Ahmad et al., 2005), and iridoids (Ahmad et al., 2008) exhibiting manifold pharmacological activities. This species exhibits nematicidal (Sultana et al., 2010), anti-inflammatory, and inhibitory activity against butyrylcholinesterase (Bukhari et al., 2016). Although B. crispa is very important medicinally, not much is known about its standardized parameters concerning habitat specificity. Nafees et al. (2022) worked on the Pharmacognostic aspect of B. asiatica leaves but significantly less work has been done for B. crispa. So, work concerning its Pharmacognostic parameters will help to establish the monograph of this plant. Further, phytochemical analysis and pharmacological screening aid in establishing the monograph of this species.
Materials and Methods
Site selection and plant material
Himachal Pradesh, India has potential mineral wealth having appreciable limestone occurrences at various locations. The limestone deposits of the Dharamkot area in District Kangra, Himachal Pradesh, India have been identified by the Geological Survey of India (Srikantia et al., 1998; Jreat, 2004; Bhat et al., 2009). B. crispa Benth. is inhabited under these limestone deposits of the Dharamkot region of Dharamshala, Himachal Pradesh, India is selected for the current study as photographed in Figure 1A (a). The herbarium sample of B. crispa was prepared and authenticated by the CSIR-National Institute of Science Communication and Policy Research, New Delhi, India having reference no. NIScPR/RHMD/Consult/2023/4507-08.
Soil microstructure
The rock samples were crushed and sieved to a particle size ≤ 80 µm and further subjected to Scanning electron microscopy (SEM) examination. The test was conducted at varying magnifications ranging from 3000x to 10000x (Amira et al., 2020).
Chemical analysis of soil
Chemical analysis of soil samples was carried out adhering to Bureau of Indian Standards (BIS) IS 1760 (Part 1 to Part 3): 1991. Loss on ignition (LOI) has been determined in calcareous soil to predict volatile impurities in the sample. The percentage of CaO, MgO, SiO2, Fe2O3, Al2O3, and CaCO3 have been measured as per methods prescribed by BIS (Dasgupta, 1985; Bureau of Indian Standards, 1991).
Detection of oxalic acid and histochemical localization of calcium oxalate crystals
Thin leaf epidermal strips encompassing trichomes were immersed in glacial acetic acid solution (5%v/v) for 30 minutes. After incubation sections were thoroughly washed with distilled water and treated with 5%w/v AgNO3 for 15 minutes followed by washing with distilled water. Thin sections were then treated with a saturated solution of dithiooxamide (silver rubeanate staining) containing ammonia (2 drops per 100 mL of 28% ammonia) and washed with ethanol and water. Black-coloured trichomes were observed under a light microscope indicating the presence of insoluble calcium oxalate and soluble oxalic acid (Fu et al., 2006; De Silva et al., 1996).
Root fungal associations
The roots of B. crispa inhabited on calcareous soil were collected and cleaned thoroughly to remove the attached debris and fixed in formalin: acetic acid: alcohol solution (5:5:90). Roots were then washed gently with distilled water and cut into small fragments (about 1 cm) and cleared by giving subsequent washings with 10% KOH acidified with 5N HCl. Ultimately, the roots underwent an overnight staining process with trypan blue and a subsequent bleaching process with alkaline hydrogen peroxide before acidification. Fungal structures were then recorded using a light microscope (Olympus Magnus, CH20 i) (Muthukumar et al., 2016).
Pharmacognostic evaluation
Macroscopy
Fresh and disease-free parts of the Himalayan butterfly bush were used to examine the external diagnostic characteristics of the plant.
Microscopy
Transverse sections (TS) of plant material encompassing leaves, stems and, roots were cut with the help of a sharp blade and subjected to further treatment using chemo-microscopic reagents. Thoroughly cleaned & stained thin sections were then examined under the light microscope. Powder microscopy of aerial parts was conducted after removing plant pigments and finally observed under a light microscope and photographed (Khandelwal, 2008; Ruzin, 1999).
SEM analysis was also performed to characterize stomata, trichomes, and calcium oxalate crystals and photographed using Thermo Scientific Phenom ProX scanning electron microscope. The samples were fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS) and incubated overnight at 4˚C. After primary fixation, secondary fixation was performed using 1% osmium tetroxide in PBS for 1-2 hours at room temperature and dried using acetone. Finally, the dried plant samples were mounted onto SEM stubs using conductive adhesive (Song et al., 2020).
Physicochemical parameters
Several parameters viz. foreign organic matter, moisture content, extractive value, total ash value, foaming index, and swelling index, were determined under standard guidelines. Preliminary phytochemical investigations have also been conducted to detect the presence of primary and secondary metabolites in the Himalayan butterfly bush (Majid et al., 2021; Khatoon et al., 2006).
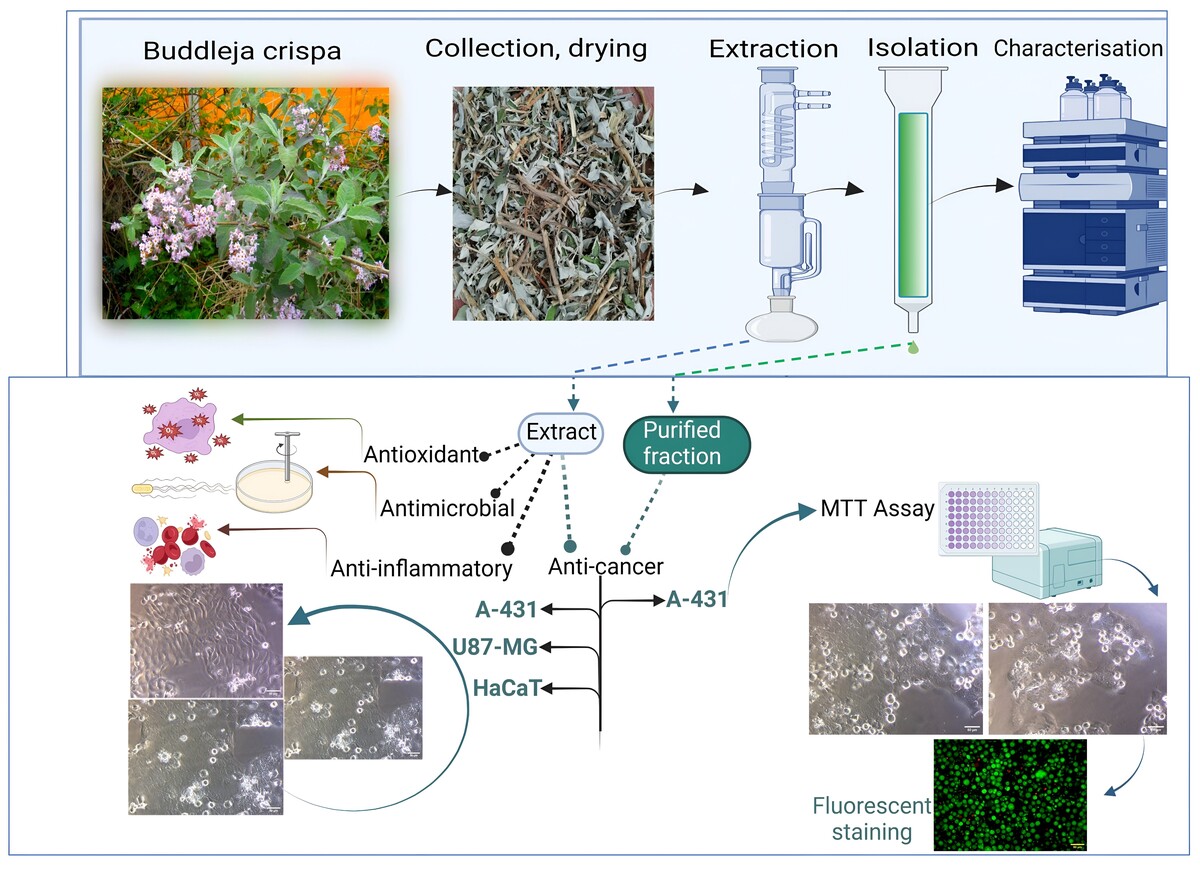
Isolation
Thoroughly dried aerial parts were subjected to soxhlation using ethanol and the extract was obtained. The crude ethanolic extract of B. crispa (BE) was further fractioned via column chromatography involving 100-200 silica gel as a stationary phase. The column was eluted using Chloroform: Methanol: Water (8:0.1:0.05 to 8:8:2) and fractions were collected. Around 20 fractions were collected and identified via TLC. Fractions (B22 to B24) corresponding to similar Rf are mixed accordingly. This fraction was kept in a refrigerator overnight for the formation of precipitates and then centrifuged. The sediments were carefully removed and subsequently washed with solvent (Figure 4a-b. Finally, the product was dried and subjected to liquid chromatography for detailed phytochemical analysis. Agilent HPLC coupled with a UV-vis was used for qualitative analysis. The separation was achieved on the C18 column. An optimized gradient mobile phase consisting of 0.1 % formic acid in water (solvent A) and acetonitrile (solvent B) delivered at 1.2 mL/min (flow rate).
Pharmacology
In-vitro Anti-oxidant activity (TAC, FRAP)
To determine in vitro antioxidant potential of B. crispa, total antioxidant capacity (TAC) and ferric reducing antioxidant power assay (FRAP) were performed on crude ethanolic extract.
The protocol outlined by Jan et al. (2013) was followed to evaluate the total antioxidant capacity of the BE and the absorbance results were compared with standard (ascorbic acid). The reducing power of BE was determined by the methodology described by Rajasekhar et al. (2009). The absorbance was measured at 700 nm in a UV-VIS spectrometer (Systronics double beam-UV-2201). Ascorbic acid at various concentrations (20 to 100 μg/mL) was used as standard.
In-vitro Anti-microbial activity
Well and disc diffusion assays were performed involving Escherichia coli, Streptococcus mutans, Staphylococcus aureus, and MICs were calculated accordingly. For well diffusion assay methodology of Sapkota et al. (2020) & Singh et al. (2013) was adopted and a disc diffusion assay was employed as per the protocol outlined by Manandhar et al. (2019) & Arullappan et al. (2009).
In-vitro Anti-inflammatory activity
Involving protein denaturation assay, the anti-inflammatory activities of the B. crispa ethanolic extracts were determined using a modified BSA assay (bovine serum albumin) mentioned by Williams et al. (2008). For the membrane stabilization assay, the protocol outlined by Sikder et al. (2010) & Yesmin et al. (2020) was followed and the percentage inhibition was calculated.
In-vitro Anti-cancer potential
Involving MTT assay (colorimetric assay), the anticancer potential of B. crispa crude extract was evaluated on the skin (A-431, HaCaT) and brain cancer (U87-MG) cell lines. The reduction of tetrazolium salts is one of the most reliable methods to examine cell proliferation. MTT assay measures the cell proliferation rate and conversely, when metabolic events lead to apoptosis or necrosis, the reduction in cell viability (Popovici et al., 2021; Gerlier et al., 1986; Mosmann, 1983). The purified fraction obtained was also tested for A-431 cell lines via MTT assay and fluorescence microscopy via acridine orange/propidium iodide staining (Bank, 1988; Liu et al., 2015; Ogunc et al., 2017; Shahruzaman et al., 2019).
Results
Soil microstructure
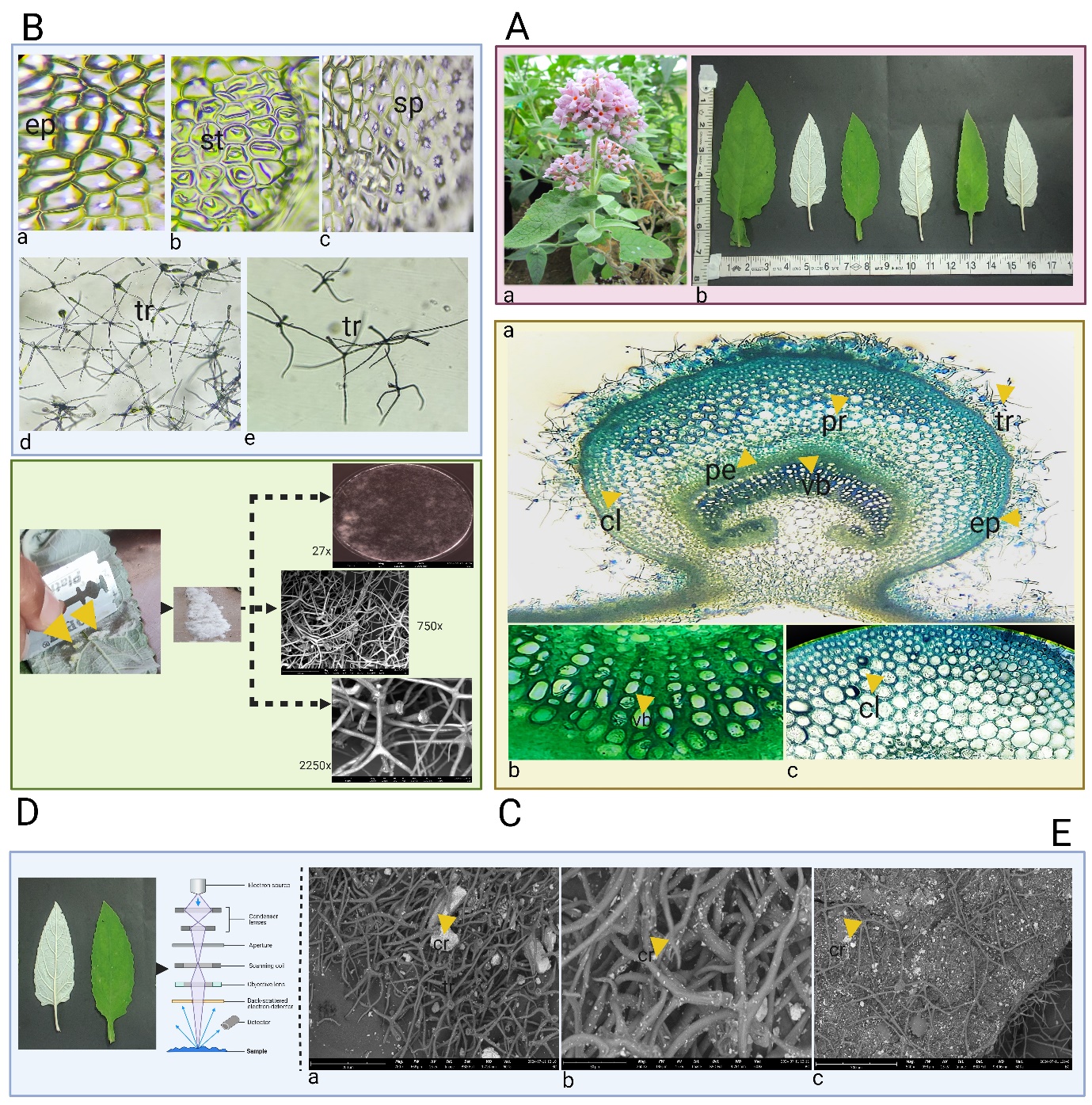
The observations of the calcareous soil microstructure using the SEM have been reported in Figure 1A (b-c). The sample showed compact morphology having grains of calcite and fine particles.
Chemical analysis of soil
The standard methodology via gravimetry and titrimetric as mentioned in IS 1760 (Part 1 to Part 3): 1991 was followed. The results for loss on ignition and percentage of other elements have been tabulated in Table 1. The detection of CaO (27.95) and CaCO3 (49.75) in soil confirms that it is rich in calcium. It was also found that an appreciable amount of Al2O3, Fe2O3, SiO2, and MgO elements existed in the sample.
Table 1
Chemical analysis of soil.
| Element | Content (% age) | Reference (BIS, 1991) |
|---|---|---|
| LOI | 40.79 | Part 1- Determination of loss on ignition |
| SiO2 | 7.50 | Part 2- Determination of silica |
| Fe2O3 | 1.27 | Part 3- Determination of iron oxide, alumina, calcium oxide, and magnesia |
| Al2O3 | 0.31 | |
| CaO | 27.95 | |
| MgO | 19.11 |
Detection of oxalic acid and histochemical localization of calcium oxalate crystals
Histochemical data revealed that oxalate ions are majorly located in trichomes of B. crispa as illustrated in Figure 1B (a-b). Further, the silver rubeanate staining method confirms the histochemical localization of calcium in trichomes as indicated by the presence of black deposits illustrated in Figure 1C (a-c).
Root fungal associations
Intracellular vesicles were observed in the roots of B. cripsa, indicating the presence of arbuscular mycorrhizal fungus Figure 1D (a-c).
Figure 1
Figure 1A. Calcareous soil microstructure (SEM). a. B. crispa inhabited on limestone deposits b-c. Images under 3000 x, 10000 x
Figure 1B. Detection of oxalic acid. a. Before localization b. After localization- black color retained in trichomes (marked by arrows)
Figure 1C. Silver rubeanate staining. a. Before staining b-c. After staining-The black deposits in the trichomes indicate the detection of calcium (marked by arrows)
Figure 1D. Root fungal association. a-c. Presence of vesicles

Pharmacognostic studies
The results of all the parameters above for pharmacognostic studies have been presented and discussed.
Macroscopy
The results of various macroscopic characteristics viz. color, odour, taste, fracture, touch, etc. of the B. crispa have been provided in Figure 2A (a-b) and Table 2.
Figure 2
Figure 2A. Macroscopic attributes. a. B. crispa plant b. Leaf morphology
Figure 2B. Representing leaf characteristics. a. Epidermal cells (ep) b. Stomatas (st) c. Spherophides (sp), d-e. Trichomes
Figure 2C. Transverse section of leaf. a. TS representing collenchyma (cl), parenchyma (pr), epidermis (ep), pericyclic fibers (pe), trichomes (tr) b. Vascular bundles (vb) c. Collenchyma (cl)
Figure 2D. Woolly epidermis under SEM (trichome networking)
Figure 2E. Tricomes in view. a-c. Arrows (yellow marked) showed abundantly scattered crystals in trichomes and epidermal cells
Microscopic studies
Paracytic stomata (Rubiaceous), non-glandular stellate trichomes (tetraradiate), and sphaeraphides (calcium oxalate crystals) embedded in parenchymatous cells are the major diagnostic features of the leaf as represented in Figure 2 (A-E). The scrapped woolly layer of the leaf showed complex trichome networking under SEM as revealed in Figure 2D. Scattered calcium oxalate crystals were detected in trichomes and other epidermal tissues when observed under SEM as represented in Figure 2E. Epidermis, cortex, medullary rays, endodermis, and pith were the main attributes reported in the stem as illustrated in Figure 3B (a-d). The transverse section of the root revealed the thick-walled polygonal epidermis, cork cells, medullary rays (arranged parallelly), collenchymatous layer, and vascular bundles as shown in Figure 3C (a-d). Powder characteristics include stomata, trichomes, thick-walled epidermal cells, and scattered calcium oxalate crystals (Figure 3D).
Figure 3
Figure 3A. Stem and root morphology of B. crispa.
Figure 3B. Microscopy of stem a. TS of stem showing epidermis (ep), cortex (ct), endodermis (ed), medullary rays (mr), vascular bundles (vb), pith (pt).
Figure 3C. Microscopy of root a. TS of root showing pith (pt), medullary rays (mr), epidermis (ep), cork cells (cr) collenchyma (cl), vascular bundles (vb).
Figure 3D. Powder characteristics of aerial parts. a. Epidermal cells (ep), b. Trichomes (tr), c. Crystals (cs), d. Fibers (fr).

Other standardization parameters
Table 3 shows the outcomes of the standardization process, and it was found that B. crispa contains more water-soluble components than alcohol, as evidenced by its alcoholic, water-soluble extractive values of 3.88% w/w and 10% w/w, respectively. The plant has 4.8% w/w of total ash, which implies inorganic components. The plant lacked mucilage as evidenced by the swelling index of 0.0 mL and the foaming index of less than 100. The value of the moisture content was found to be 8.0% w/w.
Preliminary phytochemical screening
As listed in Table 4, the B. crispa ethanolic extract's preliminary phytochemical screening revealed the presence of alkaloids, tannins, flavonoids, proteins, iridoids, and steroids. The phytoconstituents were known to exhibit potent pharmacological activities. The presence of iridoids in the present work is further supported by the results of Ahmad et al. (2008).
Table 4
Results of preliminary phytochemical screening.
+ve: positive, -ve: negative
Isolation
A purified fraction (B22-B24) was obtained via column chromatography and further processed to obtain off-white powder which was screened via liquid chromatography. Recorded HPLC-PDA profiling confirms that B22-B24 contained two major compounds eluted at Rt. 3.00 (14.82%) & 3.23 (82.49%) as represented in Figure 4c.
Pharmacology
In-vitro Anti-oxidant activity
The ethanolic extract of B. crispa showed an absorbance of 0.341±0.009 at 100 μg/mL when compared with ascorbic acid 0.472±0.007 at the same concentration in total antioxidant capacity as shown in Figure 5A(a). In the reducing power assay, the BE showed absorbance of 0.243±0.007 at 100 μg/mL in comparison with ascorbic acid 0.467±0.010 at the same concentration Figure 5A(b).
In-vitro Anti-microbial activity
When evaluated against Escherichia coli, Streptococcus mutans, and Staphylococcus aureus the extract showed MIC of 62.5 μg/mL, 31.25 μg/mL, and 125 μg/mL respectively in agar well diffusion assay. The antibacterial activity of BE was also determined by disc diffusion assay at concentrations ranging from 250 mg/mL to 1000 mg/mL. The extract showed promising results having a zone of inhibition (mm) ranging from 12.333±1.154 (E. coli), 6.333±0.577 (S. mutans), 16±2.645 (S. aureus) at the highest concentration (1000 mg/mL).
In-vitro Anti-inflammatory activity
A protein denaturation assay was conducted for the determination of anti-inflammatory activity of ethanolic extract. Concentrations (BE and aspirin) ranging from 25 to 125 µg/mL were tested and the results indicated that the extract showed an IC50 value of 137.13 µg/mL in comparison with aspirin which showed an IC50 value of 13.194 µg/mL as represented in Figure 5A(c-d). Membrane stabilization assay was also performed on BE and the results were compared using aspirin at various concentrations (25 to 250 µg/mL). Extract was found to be pharmacologically active and showed an IC50 value of 106.956 µg/mL in comparison with aspirin which showed an IC50 value of 21.846 µg/mL as illustrated in Figure 5A(e-f).
In-vitro Anti-cancer potential
The ethanolic extract of B. crispa was evaluated against the skin (A-431, HaCaT) and brain (U87-MG) cancer cell lines at doses ranging from 25 to 800 µg/ml. BE showed cytotoxicity against A-431 and U87-MG with an IC50 of 766.675 µg/mL, and 744.23 µg/mL respectively. In HaCaT cell lines, IC50 was not achieved even at the highest concentration of 800 µg/mL. B. crispa ethanolic extract inhibited the proliferation of tumor cells in a dose-dependent manner in A-431 and U87-MG; the detailed results are represented in Figure 5(B-D). Isolated fraction (B22-B24) was found to have IC50 of 485.04 μg/mL and showed cytotoxicity against A-431 cell lines when compared with untreated and standard in MTT assay. The cell lines were tested over a range of 15.625 to 500 μg/mL as shown in Figure 5B(c). Further, fluorescence microscopy via acridine orange and propidium iodide was also conducted to determine viable cell count and the fluorescent micrographs obtained are represented in Figure 5B(d). The isolated fraction (B22-B24) significantly (P<0.05) inhibited the proliferation of A-431 in a dose-dependent manner.
Figure 5
Figure 5 A. In vitro antioxidant and anti-inflammatory activity. a. TAC of BE b. FRP of BE c. Protein denaturation assay of aspirin d. Protein denaturation assay of BE e. Membrane stabilization assay of aspirin. f. Membrane stabilization assay of BE.
Figure 5 B. In vitro anti-cancer activity on A-431 cell lines. a. A-431 untreated group. b. Cytotoxicity assessment BE in A-431. c. Cytotoxicity assessment B22-B24 in A-431. d. Cytotoxicity assessment BE22-BE24 in A-431 involving acridine orange and propidium iodide showing viable (green), necrotic (red) cells. e. % viability v/s concentration plot of BE22-BE24 in A-431. f. % viability v/s concentration plot of BE in A-431.
Figure 5 C. In vitro anti-cancer activity on U87-MG cell lines. a. Untreated group b. Cytotoxicity assessment BE in U87-MG. c. % viability v/s concentration plot of BE in U87-MG.
Figure 5 D. In vitro anti-cancer activity on HaCaT cell lines. a. Untreated group. b. Cytotoxicity assessment BE in HaCaT. c. % viability v/s concentration plot of BE in HaCaT.

Discussion
It is well established that biotic and abiotic factors contribute to the growth and development of plants. Soil substratum is considered one of the significant edaphic factors sustaining the life form of species. Plants possess the unique ability to adapt to a wide variety of soils and flourish under them. Plants thriving in calcareous soils have developed several mechanisms to cope with excess calcium and have an inherent ability to resist calcium. B. crispa is also found to be a lime-loving species and calcium deposition is confirmed in its several parts, particularly in trichomes and, epidermal cells. Ectomycorrhizal fungus aids in promoting tolerance in plants inhabited in calcareous soil conditions. The root fungal associations in B. crispa showed that this species developed some unique mechanisms to withstand stressful alkaline soil conditions. With the increase in the demand for herbal drugs and herbal drug products, their quality ultimately becomes a topic of great concern. The quality of herbal or natural products will directly influence their pharmacological activity. Pharmacognostic evaluation of herbal drugs seems to play a pivotal role in assuring the standardization of herbal medicines and has become a gold standard in detecting adulteration in them thereby ensuring their quality and purity. Morphological evaluation describes the superficial features of any crude drug and microscopic studies form the basis for determining its internal anatomy. Other pharmacognostic parameters viz. Ash value, moisture content determination, foreign organic matter analysis, swelling index, and foaming index aid in ensuring the quality of crude drugs. Initial phytochemical screening indicates the existence of secondary metabolites (alkaloids, sterols, tannins, flavonoids, and iridoids) in the Himalayan butterfly bush. Phytochemical analysis of isolated fraction (B22-B24) via HPLC predicted the presence of two major compounds eluted at Rt 3.00 and 3.23 respectively which might be responsible for its reported anti-proliferative activity. The crude extract showed significant antioxidant, antimicrobial, anti-inflammatory, and anti-cancer properties when evaluated in vitro.
Conclusion
This study provides comprehensive information regarding the pharmacognostic profile of B. crispa Benth, which lives on a soil substratum rich in calcium. This is the first study of its kind on the Himalayan Butterfly Bush, revealing information about its standardized characteristics and calcicolous behaviour. Phytochemical analysis revealed the presence of two possible phytoconstituents present in the isolated fraction (B22-B24) responsible for anti-cancer activity. The plant showed its therapeutic potential when screened for antioxidant, antimicrobial, anti-inflammatory, and anti-cancer properties. This study's first-hand data will support the creation of this plant's monograph and inclusion in the Pharmacopoeia.
Acknowledgment
The authors acknowledge the continuous support and encouragement from Prof. Vivek Sharma, Govt. College of Pharmacy Rohru, Himachal Pradesh, India, and the Department of Pharmaceutical Sciences, CT University Ludhiana, Punjab, India. The authors highly appreciated the SEM facilities provided by Dextrose Laboratories, Bangalore, and PBRI, Bhopal, India for adding valuable inputs to this research work.
Abbreviations
SEM: Scanning electron microscopy
LOI: Loss on ignition
BIS: Bureau of Indian Standards
WHO: World Health Organisation
PBS: Phosphate-buffered saline
CSIR: Council of Scientific & Industrial Research
BE: Ethanolic extract of B. crispa
TLC: Thin layer chromatography
B22-B24: Isolated fraction of B. crispa
BSA: Bovine Serum albumin
MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide
ESI: Electrospray ionization
HPLC-PDA: High performance liquid chromatography-Photo diode array detector
A-431: Epidermoid carcinoma cell line
HaCaT: Immortal keratinocyte cell line
U87-MG: Glioblastoma cell line
MIC: Minimum inhibitory concentration
TAC: Total antioxidant capacity
FRAP: Ferric reducing antioxidant power assay
Author Contributons
This research is done by collaborating with the authors. All the authors revised and approved the final article.
Research design, Conceptualization: AKG, RKP; Manuscript writing: RKP; Pharmacognostic evaluation: RKP, VA, VT; Extraction and isolation work: RKP, VA; Cell line work and interpretation: AA, RKP; Proofreading and editing: AKG, VT.