INTRODUCTION
Air pollution from particulate matter in Northern Thailand causes a crisis every year for the past five years, resulting in significantly increased hospitalization rates due to pulmonary diseases, particularly from December to April (Ngamsang, Amnuaylojaroen, Parasin, & Pimonsree, 2023; Outapa & Ivanovitch, 2019). This impact is especially pronounced in at-risk populations such as the elderly, children, and pregnant women. The main components of particulate matter include organic ions, organic chemicals, transition metals, and polycyclic aromatic hydrocarbons (PAHs) (Chantara, 2012; Nuchdang et al., 2023; Othman, Latif, & Mohamed, 2016). Among these components, particles with an aerodynamic diameter of 10 microns or less are referred to as Particulate Matter-10 (PM10).
PM10 can enter the lungs and be deposited in the lung cells, enhancing lung cell injury and inflammation (Tamagawa et al., 2008). Metal and organic particulate components enhance the formation of reactive oxygen species (ROS) with subsequent potential induction of oxidative stress (Donaldson et al., 2003). Moreover, PM10 can stimulate secondary ROS generated as part of an inflammatory response. Given that an increased exposure to PM10 was proportional to the increased risk of pulmonary diseases, lung cancer (Cui, Huang, Han, Song, & Chen, 2015; Hamra et al., 2014; Zhou, Li, & Hu, 2017) and heart diseases (Kim et al., 2021; Stafoggia et al., 2022). According to the toxicity of PM10, the World Health Organization (WHO) recommended safety levels should be limited to lower than 45 µg/m³ in a 24-hour period. Unfortunately, the exceeded level has appeared in Chiang Rai from February to April every year for the past five years. Thus, studies aimed at the prevention or improvement of the adverse effects caused by PM10 are important to mitigate these problems.
Polygonum odoratum Lour. is a biennial plant that belongs to the family Polygonaceae. Its leaves are green and slender, while the stems are red. It has traditionally been used in Thai medicine as an antiflatulence agent. Several studies have reported various bioactive compounds in P. odoratum, including alkaloids, rutin, quercetin, tannins, and quinones. Additionally, its pharmaceutical properties have been reported in antibacterial, antifungal, antioxidant, anti-inflammatory, anti-osteoporosis, and anticancer activities (Khuayjarernpanishk et al., 2022; Pawłowska, Strawa, Tomczyk, & Granica, 2020; Sungkamanee, Wattanathorn, Muchimapura, & Thukham-Mee, 2014). Several years ago, our studies reported on the bioactive compounds of this plant, confirming its richness in phenolics and flavonoids, especially quercetins (Chansiw, Champakam, Chusri, Pangjit, & Srichairatanakool, 2022). The leaf extracts of this plant exhibit strong antioxidant, anti-inflammatory, and anti-microbial activities (Chansiw et al., 2019, 2018; ) (Okonogi et al., 2016). In the present study, we aim to evaluate the protective effect of P. odoratum leaf extract (PO) on PM10-induced human lung cell injury, using PM10 collected from Chiang Rai, Thailand.
MATERIALS AND METHODS
Cell culture
Human lung epithelial cell line (A549) was purchased from the American Type Culture Collection (ATCC, CCL-185TM, VA, USA) and was cultured in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% (v/v) fetal bovine serum (FBS) with 100 U/mL penicillin and 100 µg/mL streptomycin, and then maintained at 37 OC in a 5% CO2 incubator. The cells were harvested when 70-80% confluent.
Plant extract preparation
Fresh Polygonum odoratum Lour. was collected between July and September 2021 from a supplier cultivating the plant in a local area of Chiang Rai, Thailand. The plant was prepared and deposited at the Medicinal Plant Innovation Center of Mae Fah Luang University (Herbarium number: MD2018080001-1). For extract preparation, the fresh leaves were cleaned and dried using the shade-drying method, yielding a final ratio of 10:1. Subsequently, fine powder was prepared using a mechanical mill. Fifty grams of the dried powder were extracted with 50% ethanol for 72 hours using an orbital shaker at room temperature. The extract was then filtered through Whatman No. 1 filter paper. The filtrate was evaporated and dried using a freeze dryer (Labconco) to obtain PO. The extract was stored in dark vials and kept in a freezer at -20°C until the experiment. The chemical profile of the extract was previously reported by Chansiw and colleagues (Chansiw et al., 2022).
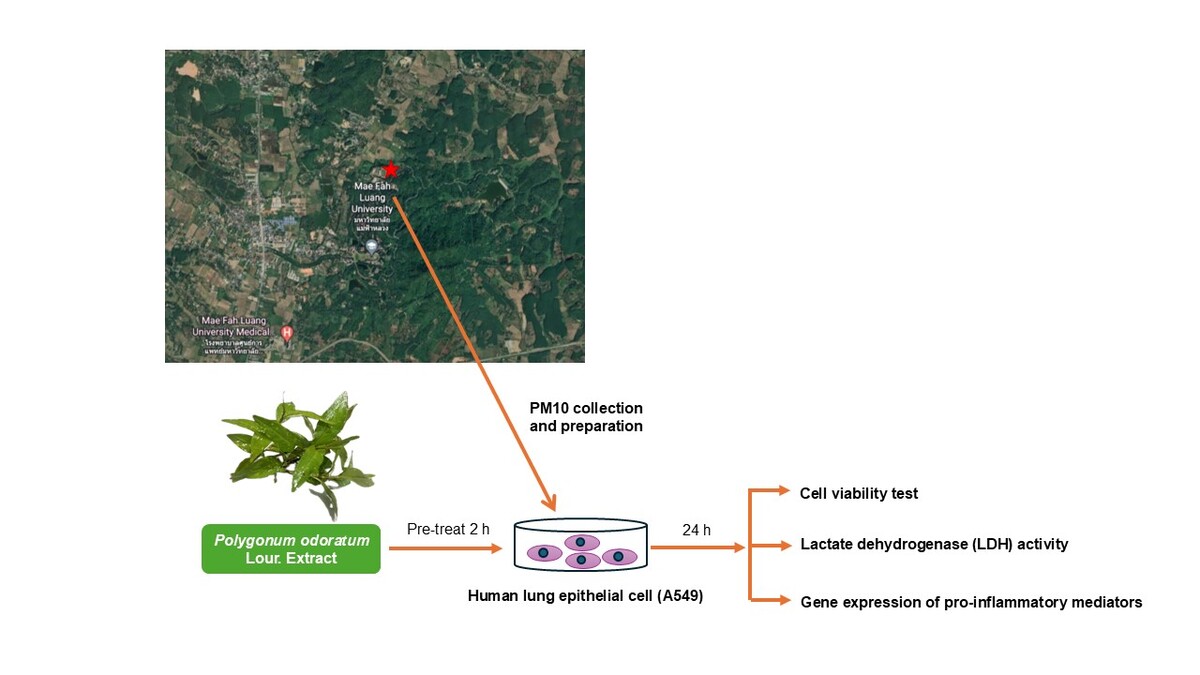
Particulate Matter 10 (PM10 Collection and Preparation)
PM10 samples were collected at the Mae Fah Luang University monitoring station in Chiang Rai, Thailand, from January to April 2022. The samples were collected on quartz fiber filters using a high-volume air sampler (TISCH Environmental). The weight of the PM10 was determined using the gravimetric method. The samples were then extracted using a mixture of acetone and hexane (1:1 ratio). They were sonicated in an ultrasonicator for 1 hour at room temperature and evaporated under a fume hood. Finally, the resulting powder was dried at 105°C for 30 minutes and stored in a desiccator for at least 24 hours to obtain PM10.
Cytotoxicity study
We investigated the appropriate concentrations of PO and PM10 by measuring cell viability using the MTT assay (Kumar, Nagarajan, & Uchil, 2018). A549 cells (1×10⁴ cells/well) were first seeded in a 96-well plate and incubated for 12–18 hours. Afterward, the medium was removed, and the cells were rinsed twice with serum-free FBS medium. Subsequently, the cells were exposed to a serum-free FBS medium containing various concentrations of PM10 or PO extract (0–400 µg/mL) for 24 hours. Following the incubation period, the medium was removed, and MTT solution (5 mg/mL in PBS) was added, followed by a 2-hour incubation at 37°C. DMSO solution was then added to dissolve the formazan crystals. Finally, the absorbance was measured at a wavelength of 540 nm. Untreated cells were used as the control group (representing 100% viable cells) to calculate the percentage of viable cells after treatment
Evaluation of PO protective effect
The protective effect of PO extract was assessed using the MTT assay as described above. Briefly, cells (1×10⁴ cells/well) were seeded in a 96-well plate and incubated for 12–18 hours. Subsequently, the cells were washed with serum-free FBS medium. Following this, the cells were pre-treated with various concentrations of PO extract (0, 50, 100, and 200 µg/mL) for 2 hours and then co-treated with PM10 (100 µg/mL) for 24 hours. Afterward, the medium was removed, and MTT solution was added for 2 hours. The formazan crystals were dissolved in DMSO, and absorbance was measured at a wavelength of 540 nm.
Measurement of Lactate dehydrogenase (LDH activity)
A549 cells (1×10⁶ cells/well) were seeded in a 6-well plate and incubated for 12–18 hours. Subsequently, the medium was removed, and the cells were rinsed with serum-free FBS medium. Following this, the cells were pre-treated with various concentrations of PO extract (0, 50, 100, and 200 µg/mL) for 2 hours and then co-treated with PM10 (100 µg/mL) for 24 hours. After the incubation period, the medium was collected and centrifuged at 12,000 rpm for 10 minutes. The supernatant was then used to measure LDH activity using the Lactate Dehydrogenase Activity Assay Kit (Sigma-Aldrich, USA), following the manufacturer’s instructions.
Gene expression of pro-inflammatory mediators by RT-qPCR
Gene expression was analyzed using quantitative real-time polymerase chain reaction (RT-qPCR). Total RNA from A549 cells (2×10⁵ cells/well) was extracted using the PureLink™ RNA Mini Kit (Invitrogen Life Sciences, Carlsbad, CA) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from 1 μg of total RNA using the SensiFAST™ cDNA Synthesis Kit (Bioline Reagent, USA). RT-qPCR was performed using the SensiFAST™ SYBR No-ROX Kit (Bioline Reagent, USA) in a reaction mixture containing SYBR Green 2× PCR Master Mix, the cDNA template, and forward and reverse primers. The PCR protocol consisted of an initial hold at 95 °C for 2 minutes, followed by a two-step PCR program of 95 °C for 15 seconds and 58 °C for 30 seconds for 39 cycles (Chansiw et al., 2022). The relative mRNA expression of pro-inflammatory mediators, including interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-13 (IL-13), and interleukin-17 (IL-17), was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the 2ΔΔCT method. The primer sequences used in this experiment are shown in Table 1 (Ko et al., 2020).
Table 1
Primer sequences for pro-inflammatory mediator expression in real-time PCR.
Statistical analysis
Data are expressed as mean ± S.E.M. Treatment effects were statistically analyzed using a one-way analysis of variance (ANOVA). When ANOVA indicated significant differences, comparisons between means were performed using the post hoc Tukey’s test. A p-value of < 0.05 was considered statistically significant. All statistical analyses were conducted using the GraphPad Prism 9 software (San Diego, CA, USA).
RESULTS
PM10 daily concentration
Figure 1
The graph illustrates the amount of particulate matter-10 (PM10) in the air at Mae Fah Luang University station from January to April 2022. PM10 was collected using a High-Volume Air Sampler and weighed using the gravimetric method.

The results of measuring particulate matter (PM10) in the air from January to April showed values ranging from 23 to 122 µg/m³, with an average of 57 µg/m³have been instances of exceeding the standard safety levels of WHO air quality guidelines until the end of February to April (Figure 1).
Cytotoxicity of PO and PM10
All concentrations of PO were not toxic to A549 cells as shown in Figure 2A. Thus, we selected PO at 50, 100 and 200 µg/mL for the next experiments. As shown in Figure 2 B, higher concentrations of PM10 exerted cytotoxicity to A549 cells. The cell viability of A549 declined in a dose-dependent manner. At the highest concentration (400 µg/mL), the cell viability was 48.40 ± 0.88 % and IC50 was 83.33 ± 2.19 µg/mL
Figure 2
Cell viability of human lung epithelial cell line (A549) after treatment PO (A) or PM10 (B) at various concentrations for 24 hours. The viability was measured by MTT assay. Data obtained from three independent experiments are expressed as mean ± S.E.M. *p <0.05 when compared to the untreated cells.

Protective effect of PO on PM10-induced lung cell injury
Effect on cell viability
As shown in Figure 3, PM10 at concentration of 100 µg/mL significantly reduced cell viability when compared with unexposed cells. Whereas treatment with PO (100 and 200 μg/mL) significantly increased the cell viability of lung cells in dose-dependent manner.
Figure 3
Cell viability of the human lung epithelial cell line (A549) after pre-treatment with PO at various concentrations for 2 hours, followed by co-treatment with PM10 (100 µg/mL) for 24 hours, was measured using the MTT assay. Data obtained from three independent experiments are expressed as mean ± S.E.M. *p <0.05 when compared to the control cells (unexposed PM10). # p < 0.05 when compared to the untreated cells.

Effect on lactate dehydrogenase (LDH activity)
As shown in Figure 4, PM10 can cause lung cell injury that showed significantly increased LDH secretion when compared with the control cells. The activity increased to 35.15 ± 1.29 U/L. Interestingly, all concentrations of PO significantly decreased lung cell injury in dose-dependent manner.
Figure 4
Lactate dehydrogenase (LDH) activity in the human lung epithelial cell line (A549) was measured after pre-treatment with PO at various concentrations for 2 hours, followed by co-treatment with PM10 (100 µg/mL) for 24 hours. The cell supernatant was harvested, and LDH activity was analyzed using a colorimetric-based method. Data obtained from three independent experiments are expressed as mean ± S.E.M. *p <0.05 when compared to the control cells (unexposed PM10). #p< 0.05 when compared to the untreated cells.

Effect PO on PM10-induced pro-inflammatory gene expression
As shown in Figure 5, the relative gene expression of IL-1β (Figure 5A), IL-6 (Figure 5B), IL-8 (Figure 5C), IL-13 (Figure 5D), and IL-17 (Figure 5E) were significantly increased in PM10-induced A549 lung cells compared with control cells (unexposed PM10). On the other hand, co-treatment with the PO extract at all concentrations trended to decrease the expression all inflammatory cytokines in a dose-dependent response (p < 0.05). Moreover, the expression of all pro-inflammatory cytokines was significantly reduced by treatment of the cells with the PO extract at 200 μg/mL.
Figure 5
Gene expression of pro-inflammatory cytokines,including IL-1β (A), IL-6 (B), IL-8 (C), IL-13 (D), and IL-17 (E), in the humanlung epithelial cell line (A549) was analyzed after pre-treatment with PO atvarious concentrations for 2 hours, followed by co-treatment with PM10 (100µg/mL) for 24 hours. The expression of pro-inflammatory genes was investigatedusing quantitative real-time PCR. Data obtained from three independentexperiments are expressed as mean ± S.E.M. *p <0.05 whencompared to the control cells (unexposed PM10). #p< 0.05 when compared to the untreated cells.

DISCUSSION
In the present study, we collected PM10 from January to April 2022 at Mae Fah Luang University station. It was found that the concentration of PM10 exceeded the WHO standard level from the end of February to April. Additionally, the collected PM10 was extracted for studying in a cell culture model. The results demonstrated that increased concentrations of PM10 at 100 µg/mL caused lung cell injury by decreasing cell viability and activating LDH secretion. Furthermore, it stimulated gene expression of pro-inflammatory cytokines including IL-1β, IL-6, IL-8, IL-13, and IL-17. However, treatment of the lung cells with PO helped neutralize the toxicity and reduce the expression of pro-inflammatory genes.
Particulate matter 10 (PM10) is a complex mixture of toxicological substances that can enter the lungs, deposit in the airways, and penetrate peripheral lung cells. Previous studies have shown that forest and agricultural waste burning is a significant contributor to air pollution in northern Thailand. Particulate matter from forest fires contains polycyclic aromatic hydrocarbons (PAHs), which induce oxidative stress, inflammation, and cancer cell metastasis (Pintha, Chaiwangyen, Yodkeeree, Suttajit, & Tantipaiboonwong, 2021). Additionally, PM10 has been shown to enhance cellular oxidative stress (Radan et al., 2019), activating the secretion of pro-inflammatory mediators such as IL-1β, IL-6, IL-8, and TNF-α (Fujii, Hayashi, Hogg, Vincent, & Eeden, 2001; Hetland et al., 2004; Ishii et al., 2004). This condition contributes to the development of airway diseases, including acute lung injury, COPD, chronic bronchitis, and asthma.
Polygonum odoratum Lour. is an indigenous plant that revealed medicinal properties, including the treatment of flatulence, antioxidant, and anti-bacterial activities. From our previous studies, the leaf extract of P. odoratum (PO) contains high amounts of phenolic compounds and flavonoids and we can found that a major active compound was quercetin (Chansiw et al., 2022). It exerts antioxidant and anti-inflammatory properties. Many studies have reported that quercetin protects against lung function alteration, leukocyte recruitment, and pro-inflammatory gene expression in lipopolysaccharide- (Gerin et al., 2016; Huang, Zhong, & Wu, 2015; Takashima et al., 2014) and cigarette smoke induced acute lung injury in animal models (Araújo et al., 2020). The molecular mechanism of quercetin for decreasing ROS-induced oxidative stress and inflammation involves suppressing the mRNA and protein expression of nicotinamide adenine dinucleotide phosphate oxidase 2 (NOX2). It also suppresses the nuclear translocation of nuclear factor kappa B (NF-κB) and reduces the levels of inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6 in LPS-induced lung inflammation (Sul & Ra, 2021). These findings strongly suggest that PO contains a potential bioactive compound that possesses anti-inflammatory activity, through suppression of pro-inflammatory gene expression, in PM10-induced lung cell injury. However, the study in molecular mechanism and animal models requires further elucidation.
CONCLUSION
This study demonstrates that Chiang Rai Province, Thailand, experiences elevated PM10 levels annually from late February to April. High concentrations of PM10 are toxic to lung cells and induce inflammation. Therefore, Polygonum odoratum Lour. extract, with its antioxidant and anti-inflammatory properties, presents a promising alternative for treating and preventing toxicity caused by PM10 exposure. However, further investigation is required into the composition of PM10, its molecular mechanisms, and studies involving animal models.
Abbreviations
ANOVA = one-way analysis of variance
DMSO = Dimethyl sulfoxide 10
IL1-β = interleukin-1β
IL-6 = interleukin 6
IL-8 = interleukin 8
IL-13 = interleukin 13
IL-17 = interleukin 17
LDH = lactate dehydrogenase
LPS = lipopolysaccharide
MTT = 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide
NF-κB = nuclear factor kappa B
PM10 = Particulate matter
PBS = phosphate buffer saline
S.E.M = standard error of mean
TNF-α = tumor necrosis factor-α.
Author contributions
Nittaya Chansiw: Research concept and design, Collection and/or assembly of data, Data analysis and interpretation, Writing the article, Critical revision of the article, Final approval of the article. Narudol Teerapattarakan: Collection and/or assembly of data, Data analysis and interpretation. Pattranuch Chusri: Collection and/or assembly of data, Data analysis and interpretation. Weerayuth Siriratreungsuk: Collection and/or assembly of data, Data analysis and interpretation. Utthapon Issara: Collection and/or assembly of data, Data analysis and interpretation.